Marine Protected Areas (Mpas) in Management 1 of Coral Reefs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coral Reef Protection in Quintana Roo, Mexico. Intercoast #34 ______
_____________________________________________________________________________ Coral Reef Protection in Quintana Roo, Mexico. Intercoast #34 _____________________________________________________________________________ Bezaury, Juan and Jennifer McCann 1999 Citation: Narragansett, Rhode Island USA: Coastal Resources Center.InterCoast Network Newsletter, Spring 1999 For more information contact: Pamela Rubinoff, Coastal Resources Center, Graduate School of Oceanography, University of Rhode Island. 220 South Ferry Road, Narragansett, RI 02882 Telephone: 401.874.6224 Fax: 401.789.4670 Email: [email protected] This five year project aims to conserve critical coastal resources in Mexico by building capacity of NGOs, Universities, communities and other key public and private stakeholders to promote an integrated approach to participatory coastal management and enhanced decision-making. This publication was made possible through support provided by the U.S. Agency for International Development’s Office of Environment and Natural Resources Bureau for Economic Growth, Agriculture and Trade under the terms of Cooperative Agreement No. PCE-A-00-95-0030-05. INTERNATIONAL NEWSLETTER OF COASTAL MANAGEMENT Narragansett, Rhode Island, U.S.A. • #31 • Spring, 1998 Protecting the Maya Reef Intercoast Through Multi-National Survey Results Cooperation Show Diverse manage their coastal resources region- Readership By Juan Bezaury and ally. The overall goal is to take advan- Jennifer McCann tage of growing opportunities for sus- ore than 200 people tainable development, -

The Blue Paradox: Preemptive Overfishing in Marine Reserves
PAPER The blue paradox: Preemptive overfishing in COLLOQUIUM marine reserves Grant R. McDermotta,1,2, Kyle C. Mengb,c,d,1,2, Gavin G. McDonaldb,e, and Christopher J. Costellob,c,d,e aDepartment of Economics, University of Oregon, Eugene, OR 97403; bBren School of Environmental Science & Management, University of California, Santa Barbara, CA 93106; cDepartment of Economics, University of California, Santa Barbara, CA 93106; dNational Bureau of Economic Research, Cambridge, MA 02138; and eMarine Science Institute, University of California, Santa Barbara, CA 93106 Edited by Jane Lubchenco, Oregon State University, Corvallis, OR, and approved July 25, 2018 (received for review March 9, 2018) Most large-scale conservation policies are anticipated or an- This line of reasoning suggests that the anticipation of a new nounced in advance. This risks the possibility of preemptive conservation policy may give rise to a set of incentives that is resource extraction before the conservation intervention goes distinct from—but possibly just as important as—the incentives into force. We use a high-resolution dataset of satellite-based fish- arising from the policy’s implementation. While this kind of pre- ing activity to show that anticipation of an impending no-take emptive behavior has been well-documented for landowners, gun marine reserve undermines the policy by triggering an unin- owners, and owners of natural resource extraction rights, it has tended race-to-fish. We study one of the world’s largest marine not been studied in the commons. For vast swaths of the ocean, reserves, the Phoenix Islands Protected Area (PIPA), and find that no single owner has exclusive rights and so must compete against fishers more than doubled their fishing effort once this area was others for extraction. -
Global Patterns in Marine Mammal Distributions
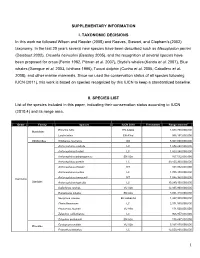
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

The Role of Marine Protected Areas in Sustaining Fisheries
The role of marine protected areas in sustaining fisheries Callum Roberts University of York, UK After World War II there was much optimism that fisheries could feed the World. But at the beginning of the 21st century, we are not so sure. Quota management of fisheries in the European Union has failed to deliver sustainability 100 80 60 40 20 Percentage of Quota Fish Stocks of Quota Percentage 0 1970 1975 1980 1985 1990 1995 2000 Year Healthy At risk In danger Data from ICES Cod decline in the Kattegat, North Sea Extinction is the ultimate in unsustainable fishing, whether or not the species of concern are targets of the fishery What is missing from fishery management? • Real provision for habitat protection and recovery • Precautionary targets • Resolute enforcement Objectives of marine reserves Maintaining ecosystem processes and services Conservation Sustaining fisheries Tree cover www.worldwildlife.org/oceans/pdfs/fishery_effects.pdf Spillover Reproduction & Dispersal Colonization & Growth Abundance Diversity What is the evidence that reserves work? Reserves all over the world show dramatic increases in spawning stocks Usually by at least 2-3 times in 5-10 years Long-term studies in New Zealand, Philippines, Florida and many other countries show strong responses to reserve protection Fish in reserves do live longer, grow larger and produce more eggs Egg production from protected fish stocks increases by much more than stock biomass Catches do increase Soufrière Marine Management Area, St. Lucia: Established 1995 35% of reef area closed -

Critical Habitats and Biodiversity: Inventory, Thresholds and Governance
Commissioned by BLUE PAPER Summary for Decision-Makers Critical Habitats and Biodiversity: Inventory, Thresholds and Governance Global biodiversity loss results from decades of unsustainable use of the marine environment and rep- resents a major threat to the ecosystem services on which we, and future generations, depend. In the past century, overexploitation of fisheries and the effects of bycatch have caused species to decline whilst coastal reclamation and land-use change—together with pollution and, increasingly, climate change—have led to vast losses of many valuable coastal habitats, estimated at an average of 30–50 percent.1 Despite advances in understanding how marine species and habitats are distributed in the ocean, trends in marine biodiversity are difficult to ascertain. This results from the very patchy state of knowl- edge of marine biodiversity, which leads to biases in understanding different geographic areas, groups of species, habitat distribution and patterns of decline. To address the data gap in our understanding of marine biodiversity and ecosystem integrity, it is crucial to establish the capacity to assess current baselines and trends through survey and monitoring activities. Only increased knowledge and understanding of the above will be able to inform ocean manage- ment and conservation strategies capable of reversing the current loss trend in marine biodiversity. A new paper2 commissioned by the High Level Panel for a Sustainable Ocean Economy increases our understanding of these trends by analysing the links between biodiversity and ecosystem functioning (including tipping points for degradation) and unpacks the link between protection and gross domes- tic product (GDP). In doing so, the paper provides an updated catalogue of marine habitats and biodiversity and outlines five priorities for changing the current trajectory of decline. -

Marine Protected Areas
Marine Protected Areas Read the passage below. How do oceans affect you? If you live far from the coast, you might think they don’t. But life on this planet depends on the oceans. They cover almost three-quarters of the planet and hold 97 percent of the Earth’s water. The phytoplankton that live on the oceans’ surface produce 70 percent of the oxygen in the atmosphere. Oceans are a vital source of food and other resources, and an economic engine for many communities. For all the oceans provide us, we haven’t always been so responsible in our stewardship. “The ocean was thought of as a dumping ground for so long,” says Caitlyn Toropova of the International Union for Conservation of Nature (IUCN). “There was a sense that there was no way we could harm it because it is so vast.” But human activities are having a negative impact on many of the world’s oceans, jeopardizing marine life, habitat, and ecosystems. These threats include overfishing or destructive fishing, coastal development, pollution and runoff, and the introduction of non-native species. Climate change is also having a big effect by causing warming seas and rising acidification. The realization that something needs to be done to stem or reverse the damage has led to the creation of Marine Protected Areas (MPAs). Broadly speaking, marine protected areas are regions of the ocean where human activity is limited. Specifics differ around the globe, but the United States defines a marine protected area as “any area of the marine environment that has been reserved by federal, state, tribal, territorial, or local laws or regulations to provide lasting protection for part or all of the natural and cultural resources therein.” There are approximately 5,000 designated MPAs around the world, and many more that are not officially recognized, says Toropova, the conservation group’s coordination officer for marine protected areas. -

Coral Reef Biological Criteria: Using the Clean Water Act to Protect a National Treasure
EPA/600/R-10/054 | July 2010 | www.epa.gov/ord Coral Reef Biological Criteria: Using the Clean Water Act to Protect a National Treasure Offi ce of Research and Development | National Health and Environmental Effects Research Laboratory EPA/600/R-10/054 July 2010 www.epa.gov/ord Coral Reef Biological Criteria Using the Clean Water Act to Protect a National Treasure by Patricia Bradley Leska S. Fore Atlantic Ecology Division Statistical Design NHEERL, ORD 136 NW 40th St. 33 East Quay Road Seattle, WA 98107 Key West, FL 33040 William Fisher Wayne Davis Gulf Ecology Division Environmental Analysis Division NHEERL, ORD Offi ce of Environmental Information 1 Sabine Island Drive 701 Mapes Road Gulf Breeze, FL 32561 Fort Meade, MD 20755 Contract No. EP-C-06-033 Work Assignment 3-11 Great Lakes Environmental Center, Inc Project Officer: Work Assignment Manager: Susan K. Jackson Wayne Davis Offi ce of Water Offi ce of Environmental Information Washington, DC 20460 Fort Meade, MD 20755 National Health and Environmental Effects Research Laboratory Offi ce of Research and Development Washington, DC 20460 Printed on chlorine free 100% recycled paper with 100% post-consumer fiber using vegetable-based ink. Notice and Disclaimer The U.S. Environmental Protection Agency through its Offi ce of Research and Development, Offi ce of Environmental Information, and Offi ce of Water funded and collaborated in the research described here under Contract EP-C-06-033, Work Assignment 3-11, to Great Lakes Environmental Center, Inc. It has been subject to the Agency’s peer and administrative review and has been approved for publication as an EPA document. -

Multi-Zone Marine Protected Areas: Assessment of Ecosystem and Fisheries Benefits Using Multiple Ecosystem Models X
Multi-zone marine protected areas: assessment of ecosystem and fisheries benefits using multiple ecosystem models X. Corrales, D. Vilas, C. Piroddi, J. Steenbeek, Joachim Claudet, J. Lloret, A. Calò, A. Di Franco, T. Font, A. Ligas, et al. To cite this version: X. Corrales, D. Vilas, C. Piroddi, J. Steenbeek, Joachim Claudet, et al.. Multi-zone marine pro- tected areas: assessment of ecosystem and fisheries benefits using multiple ecosystem models. Ocean and Coastal Management, Elsevier, 2020, 193, pp.105232. 10.1016/j.ocecoaman.2020.105232. hal- 03034139 HAL Id: hal-03034139 https://hal.archives-ouvertes.fr/hal-03034139 Submitted on 1 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright 1 To be re-submitted to Ocean and Coastal Management 2 Multi-zone marine protected areas: assessment of ecosystem and fisheries 3 benefits using multiple ecosystem models 4 Corrales, X.1,2,3; Vilas, D.1,2,4,5; Piroddi, C.6; Steenbeek, J.2; Claudet, J.7; Lloret, J.8; Calò, 5 A.9,10; Di Franco, A.9,11; Font, T.8; Ligas, A.12; Prato, G.13; Sahyoun, R.13; Sartor, P.12; 6 Guidetti, P.9,14; Coll, M.1,2. -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc. -

Marine Protection in America's Ocean
SeaStates 2021 Marine Protection in America’s Ocean MARINE PROTECTION ATLAS SeaStates 2021 Marine Protection in America’s Ocean SeaStates 2021 Marine Protection in America’s Ocean SeaStates 2021 is a rigorous, quantitative accounting of fully and words marine reserve or marine protected area are used. Protect- strongly protected MPAs in America’s ocean updated annually by ing biodiversity in marine reserves increases the abundance and Sardines swirl amongst giant kelp off Anacapa Island, part the team at MPAtlas.org. First published in 2013, our annual reports diversity of marine life inside the reserve and typically results in of the Channel Islands National Marine Sanctuary. Federal are intended to measure US progress as a whole, and state prog- the export of marine life to surrounding areas4,5. In short, no-take and state authorities co-manage the MPA complex in the Channel Islands which includes a network of fully protected ress towards effective marine protection in their coastal waters. MPAs effectively protect marine life, can secure food resources areas surrounded by the highly protected sanctuary zone. Oceans are essential to human survival and prosperity, yet our for millions of people by exporting fish outside the MPA, and Marine Conservation Institute has recognized the Northern Channel Islands with a platinum-level Blue Parks award. activities are damaging many ecosystems and pushing numerous prevent loss of species. Strongly protected areas are those in (credit Robert Schwemmer, NOAA) critical marine species toward extinction1. Many marine biologists which extraction is quite limited and conservation benefits are high but not as high as fully protected areas. -

Guidelines for Marine Protected Areas
Guidelines for Marine Protected Areas World Commission on Protected Areas (WCPA) Guidelines for Marine MPAs are needed in all parts of the world – but it is vital to get the support Protected Areas of local communities Edited and coordinated by Graeme Kelleher Adrian Phillips, Series Editor IUCN Protected Areas Programme IUCN Publications Services Unit Rue Mauverney 28 219c Huntingdon Road CH-1196 Gland, Switzerland Cambridge, CB3 0DL, UK Tel: + 41 22 999 00 01 Tel: + 44 1223 277894 Fax: + 41 22 999 00 15 Fax: + 44 1223 277175 E-mail: [email protected] E-mail: [email protected] Best Practice Protected Area Guidelines Series No. 3 IUCN The World Conservation Union The World Conservation Union CZM-Centre These Guidelines are designed to be used in association with other publications which cover relevant subjects in greater detail. In particular, users are encouraged to refer to the following: Case studies of MPAs and their Volume 8, No 2 of PARKS magazine (1998) contributions to fisheries Existing MPAs and priorities for A Global Representative System of Marine establishment and management Protected Areas, edited by Graeme Kelleher, Chris Bleakley and Sue Wells. Great Barrier Reef Marine Park Authority, The World Bank, and IUCN. 4 vols. 1995 Planning and managing MPAs Marine and Coastal Protected Areas: A Guide for Planners and Managers, edited by R.V. Salm and J.R. Clark. IUCN, 1984. Integrated ecosystem management The Contributions of Science to Integrated Coastal Management. GESAMP, 1996 Systems design of protected areas National System Planning for Protected Areas, by Adrian G. Davey. Best Practice Protected Area Guidelines Series No. -

Oregon Marine Reserves Ecological Monitoring Plan 2012
Oregon Marine Reserves Ecological Monitoring Plan 2012 Marine Resources Program Newport, Oregon Acknowledgments: Thank you to the Redfish Rocks and Otter Rock Marine Reserve Community Teams, biological working groups, local fishermen, divers and scientific experts for their time, hard work, and contribution to the development of the monitoring plan and to this document. Individual Acknowledgments: Otter Rock Community Team Biological Working Group: Jim Burke, Terry Thompson, Paul Erskine and Gary Wise Redfish Rocks Community Team Science Working Group: Jim Golden, Dick Vander Schaaf, Dave Lacey, Brianna Goodwin, Lyle Keeler Advisors: Mark Carr, Mark Hixon, Alan Shanks, Rick Starr, Brian Tissot and the members of the Ocean Policy and Advisory Council’s Science and Technical Advisory Committee. Contributors: Alix Laferriere, Cristen Don, David Fox, Keith Matteson, Mike Donnellan, Scott Groth, Annie Pollard. Cover photo: Scott Groth. Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-7701 x 227 www.dfw.state.or.us/MRP/ www.oregonocean.info/marinereserves Oregon Department of Fish and Wildlife Table of Contents I. Introduction ............................................................................................................................................. 1 A. Monitoring Plan Purpose..................................................................................................................... 1 B. Marine Reserves: Oregon’s Policy Guidance ....................................................................................