A Three Dimensional Nerve Map of Human Bladder Trigone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

St. Lawrence School Subject
St. Lawrence School Subject - Science Class - 4 Chapter - 3 Human Body : Digestive and Excetory System ( Part - 1 ) Learn about * Digestive system * Excretory system * Healthy eating habits Digestive System The process by which food is broken down into a simpler form so that it can be easily taken in or absorbed by our body is called digestion. Many organs work together and help in the process of digestion. The mouth, food pipe, stomach, small and large intestine, liver, rectum, and anus are the main organs of the digestive system. Let us learn about them. Mouth Digestion starts in the mouth. The teeth help to break down and chew food. The chewed food then mixes with a liquid, called saliva, produced in our mouth. It makes the food softer and easier to swallow. The tongue helps in the proper mixing of saliva with the food. Food pipe The food pipe ( oesophagus ) passes the food from the mouth to the stomach. Stomach Inside the stomach, the food is broken down further into smaller pieces by churning and with the help of chemicals called digestive juices. Small intestine From the small intestine, the undigested food passes into the large intestine. The large intestine is a shorter but wider, tube - like structure, which collects the indigestible food from the small intestine. The large intestine absorbs water from this undigested food and forms waste products called faeces. Rectum Rectum is the final part of the large intestine. Faeces are stored in the rectum for a short time before being passed out through anus. Anus Faeces are removed from the body through the anus. -

Urinary Bladder – Proteinaceous Plug
Urinary bladder – Proteinaceous Plug Figure Legend: Figure 1 An eosinophilic amorphous proteinaceous plug in the bladder lumen from a male B6C3F1 mouse in a chronic study. Figure 2 A proteinaceous plug associated with other flocculent, eosinophilic material, from a male F344/N rat in an acute study. Comment: Proteinaceous plugs are commonly noted as a postmortem change resulting from an agonal secretion of accessory sex gland fluids during euthanasia. Proteinaceous plugs vary in size but can be large, filling the urinary bladder (Figure 1 and Figure 2). Microscopically, the plug is composed of a mixture of an amorphous eosinophilic material, sometimes containing desquamated epithelial cells and spermatozoa. Proteinaceous plugs by themselves have no toxicologic importance and are not precursors of calculi. Plugs may be seen with obstructive syndromes associated with bacterial inflammation. They must be differentiated from calculi. Recommendation: Proteinaceous plugs occurring alone and not associated with any pathologic lesions should be recognized as an artifact and should not be diagnosed. Occasionally, proteinaceous plugs are recognized grossly, and the pathologist should use his or her judgment to correlate the gross lesion to an artifactual proteinaceous plug. 1 Urinary bladder – Proteinaceous Plug References: Gaillard ET. 1999. Ureter, urinary bladder and urethra. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 235– 258. Abstract: http://www.cacheriverpress.com/books/pathmouse.htm Hard GC, Alden CL, Bruner RH, Frith CH, Lewis RM, Owen RA, Krieg K, Durchfeld-Meyer B. 1999. Non-proliferative lesions of the kidney and lower urinary tract in rats. -

1535190852 9 Medical Terminology Urinary.Pdf
Syrian Private University Medical Faculty Medical Terminology M.A.Kubtan , MD – FRCS Lecture 9 M.A.Kubtan Objectives After studying this chapter, you will be able to: •Name the parts of the urinary system and discuss the function of each part •Define combining forms used in building words that relate to the urinary system •Identify the meaning of related abbreviations •Name the common diagnoses, clinical procedures, and laboratory tests used in treating disorders of the urinary system M.A.Kubtan 2 Objectives Part 2 •List and define the major pathological conditions of the urinary system •Explain the meaning of surgical terms related to the urinary system •Recognize common pharmacological agents used in treating the urinary system M.A.Kubtan 3 Structure and Function The Urinary System Bladder Kidneys •Also called the excretory system •Maintains water Urinary System balance •Removes waste products from the Urethra Ureters blood by excreting them in the urine Meatus M.A.Kubtan 4 Kidneys Kidneys The kidneys are bean-shaped organs located in the retroperitoneal portion of the abdominal cavity on either side of the vertebral column. Two Primary Functions •To form urine for excretion •To retain essential substances the body needs in the process called reabsorption M.A.Kubtan 5 Parts of the Kidney Kidneys filter about 1700 kidney liters of blood daily in the average adult. medulla Parts of the kidneys •Cortex hilum -outer protective portion •Medulla -inner soft portion •Hilum -a depression located in the middle of the concave side of the kidney where blood vessels, nerves, and the ureters enter and cortex exit the kidneys M.A.Kubtan 6 Urine Production Urine is produced by filtration of: •water •sugar •creatine •salts •urea •uric acid Each kidney contains more than 1 million nephrons which are the functional units of the kidneys. -

15-1040-Junu Oh-Neuronal.Key
Neuronal Control of the Bladder Seung-June Oh, MD Department of urology, Seoul National University Hospital Seoul National University College of Medicine Contents Relevant end organs and nervous system Reflex pathways Implication in the sacral neuromodulation Urinary bladder ! body: detrusor ! trigone and bladder neck Urethral sphincters B Preprostatic S Smooth M. Sphincter Passive Prostatic S Skeletal M. Sphincter P Prostatic SS P-M Striated Sphincter Membraneous SS Periurethral Striated M. Pubococcygeous Spinal cord ! S2–S4 spinal cord ! primary parasympathetic micturition center ! bladder and distal urethral sphincter ! T11-L2 spinal cord ! sympathetic outflow ! bladder and proximal urethral sphincter Peripheral innervation ! The lower urinary tract is innervated by 3 principal sets of peripheral nerves: ! parasympathetic -pelvic n. ! sympathetic-hypogastric n. ! somatic nervous systems –pudendal n. ! Parasympathetic and sympathetic nervous systems form pelvic plexus at the lateral side of the rectum before reaching bladder and sphincter Sympathetic & parasympathetic systems ! Sympathetic pathways ! originate from the T11-L2 (sympathetic nucleus; intermediolateral column of gray matter) ! inhibiting the bladder body and excite the bladder base and proximal urethral sphincter ! Parasympathetic nerves ! emerge from the S2-4 (parasympathetic nucleus; intermediolateral column of gray matter) ! exciting the bladder and relax the urethra Sacral somatic system !emerge from the S2-4 (Onuf’s nucleus; ventral horn) !form pudendal nerve, providing -

Anatomy and Physiology Male Reproductive System References
DEWI PUSPITA ANATOMY AND PHYSIOLOGY MALE REPRODUCTIVE SYSTEM REFERENCES . Tortora and Derrickson, 2006, Principles of Anatomy and Physiology, 11th edition, John Wiley and Sons Inc. Medical Embryology Langeman, pdf. Moore and Persaud, The Developing Human (clinically oriented Embryologi), 8th edition, Saunders, Elsevier, . Van de Graff, Human anatomy, 6th ed, Mcgraw Hill, 2001,pdf . Van de Graff& Rhees,Shaum_s outline of human anatomy and physiology, Mcgraw Hill, 2001, pdf. WHAT IS REPRODUCTION SYSTEM? . Unlike other body systems, the reproductive system is not essential for the survival of the individual; it is, however, required for the survival of the species. The RS does not become functional until it is “turned on” at puberty by the actions of sex hormones sets the reproductive system apart. The male and female reproductive systems complement each other in their common purpose of producing offspring. THE TOPIC : . 1. Gamet Formation . 2. Primary and Secondary sex organ . 3. Male Reproductive system . 4. Female Reproductive system . 5. Female Hormonal Cycle GAMET FORMATION . Gamet or sex cells are the functional reproductive cells . Contain of haploid (23 chromosomes-single) . Fertilizationdiploid (23 paired chromosomes) . One out of the 23 pairs chromosomes is the determine sex sex chromosome X or Y . XXfemale, XYmale Gametogenesis Oocytes Gameto Spermatozoa genesis XY XX XX/XY MALE OR FEMALE....? Male Reproductive system . Introduction to the Male Reproductive System . Scrotum . Testes . Spermatic Ducts, Accessory Reproductive Glands,and the Urethra . Penis . Mechanisms of Erection, Emission, and Ejaculation The urogenital system . Functionally the urogenital system can be divided into two entirely different components: the urinary system and the genital system. -

The Digestive System
69 chapter four THE DIGESTIVE SYSTEM THE DIGESTIVE SYSTEM The digestive system is structurally divided into two main parts: a long, winding tube that carries food through its length, and a series of supportive organs outside of the tube. The long tube is called the gastrointestinal (GI) tract. The GI tract extends from the mouth to the anus, and consists of the mouth, or oral cavity, the pharynx, the esophagus, the stomach, the small intestine, and the large intes- tine. It is here that the functions of mechanical digestion, chemical digestion, absorption of nutrients and water, and release of solid waste material take place. The supportive organs that lie outside the GI tract are known as accessory organs, and include the teeth, salivary glands, liver, gallbladder, and pancreas. Because most organs of the digestive system lie within body cavities, you will perform a dissection procedure that exposes the cavities before you begin identifying individual organs. You will also observe the cavities and their associated membranes before proceeding with your study of the digestive system. EXPOSING THE BODY CAVITIES should feel like the wall of a stretched balloon. With your skinned cat on its dorsal side, examine the cutting lines shown in Figure 4.1 and plan 2. Extend the cut laterally in both direc- out your dissection. Note that the numbers tions, roughly 4 inches, still working with indicate the sequence of the cutting procedure. your scissors. Cut in a curved pattern as Palpate the long, bony sternum and the softer, shown in Figure 4.1, which follows the cartilaginous xiphoid process to find the ventral contour of the diaphragm. -

Squirrel Monkey Lateral Thalamus. II. Viscerosomatic Convergent Representation of Urinary Bladder, Colon, and Esophagus
The Journal of Neuroscience, November 1994, 14(11): 6796-6614 Squirrel Monkey Lateral Thalamus. II. Viscerosomatic Convergent Representation of Urinary Bladder, Colon, and Esophagus Johannes Briiggemann, Ting Shi, and A. Vaina Apkarian Department of Neurosurgery, Computational Neuroscience Program, SUNY Health Science Center at Syracuse, Syracuse, New York 13210 The response properties of 106 visceroceptive lateral tha- the thalamus. Visceral primary afferents constitute a unique lamic neurons were investigated in anesthetized squirrel population distinct from the somatic primary afferents(e.g., less monkeys. Most neurons were located in the ventral posterior than 0.5% of the pelvic afferents branch and innervate the skin lateral nucleus (VPL), and a smaller number of cells was also and viscera; HPbler et al., 1988). Also the coding strategiesused found in a variety of thalamic nuclei around VPL. Ninety by the somatic and visceral systems in the PNS are distinct. (65%) of these cells responded to distension of the urinary Somatic primary afferents have different fiber populations sub- bladder, the distal colon, and/or the lower esophagus. The serving various stimulus modalities (e.g., touch, nociception). majority of the visceral-responsive cells also had convergent In contrast, many mechanoceptive primary visceral afferents somatic and multivisceral responses (71% of the 65%). A have “intensity-coding” properties, that is, monotonic stimulus small population (6%) was visceral specific; that is, these responsecurves over both innocuous and noxious ranges(see, neurons were not activated with somatic stimuli. Visceral e.g., Senguptaet al., 1989, 1990). Intensity-coding somatic pri- responses were excitatory, inhibitory, or mixed, and most mary afferents have not been found in the normal somatic PNS. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -

Bladder Metastasis of Non-Small Cell Lung Cancer: an Unusual Cause of Hematuria
CASE REPORT / ULUSLARARASı HEMATOLOJI-ONKOLOJI DERGISI International Journal of Hematology and Oncology OLGU SUNUMU Bladder Metastasis of non-Small Cell Lung Cancer: an Unusual Cause of Hematuria O. Faruk KARATAS1, Reyhan BAYRAK2, M. Erol YILDIRIM1, Omer BAYRAK1, Ersin CIMENTEPE1, Dogan UNAL1 1 Fatih University Faculty of Medicine Department of Urology 2 Fatih University Faculty of Medicine Department of Pathology, Ankara, TURKEY ABSTRACT Approximately 2% of bladder malignancies are metastatic. The lung cancer makes metastasis sporadically to the blad- der. A-69-year-old female patient presented with a history of pain in kidneys, vomiting and hematuria. Cystoscopic examination of the patient revealed small bladder capacity and solitary lesions in the bladder wall. Thoracic comput- ed tomography scan identified multiple solid masses in the right lung. A chemotherapy regimen against epithelial tumors (Granisetron, Carboplatin, and Gemcitabine) was recommended. At the end of the 3 courses, chemotherapy regimen was stopped because of poor general health condition. She died in 9th month of the diagnosis. Key Words: Bladder metastasis, non-Small cell lung cancer ÖZET Küçük Hücreli Dışı Akciğer Kanserinin Mesane Metastazı: Nadir bir Hematüri Nedeni Mesane tümörlerinin yaklaşık olarak %2’si metastatiktir. Akciğer kanseri sporadik olarak mesane tutulumu yapmak- tadır. Altmış dokuz yaşında bayan hasta, her iki böbrek lojuna uyan bölgede ağrı, kusma ve hematüri öyküsü ile başvurdu. Hastanın sistoskopik muayenesinde azalmış mesane kapasitesi ve mesane duvarında soliter lezyonlar sap- tandı. Toraks bilgisayarlı tomografide sağ akciğerde multipl solid kitleler saptandı. Epitelyal tümörlere karşı etkili kemoterapötikler (Granisetron, Carboplatin ve Gemsitabin) önerildi. Üçüncü kürün sonunda, kemoterapi tedavisi has- tanın genel sağlık durumunun kötüleşmesi üzerine durduruldu. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -
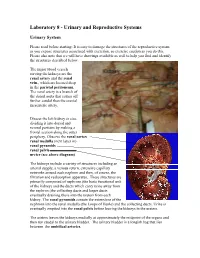
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder.