The Influence of Halloysite Content on the Shear Strength of Kaolinite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fire Retardancy of Polypropylene/Kaolinite Composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta
Fire retardancy of polypropylene/kaolinite composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta To cite this version: Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta. Fire retardancy of polypropylene/kaolinite composites. Polymer Degradation and Stability, Elsevier, 2016, 129, pp.260-267. 10.1016/j.polymdegradstab.2016.05.003. hal-02906432 HAL Id: hal-02906432 https://hal.archives-ouvertes.fr/hal-02906432 Submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Fire retardancy of polypropylene/kaolinite composites * Marcos Batistella a, c, , Belkacem Otazaghine b, Rodolphe Sonnier b, Carlos Petter c, Jose-Marie Lopez-Cuesta b a Federal University of Santa Catarina, R. Eng. Agronomico^ Andrei Cristian Ferreira, s/n e Trindade, Florianopolis, SC, CEP 88040-900, Brazil b Ecole des Mines d’Ales, Centre des Materiaux (C2MA) e Pole^ Materiaux Polymeres Avances, 6 Avenue de Clavieres, 30319, Ales Cedex, France c Federal University of Rio Grande do Sul, Av. Bento Gonçalves, 9500, Porto Alegre, CEP 91501-970, Brazil abstract In this study the influence of surface modification of kaolinite with trisilanolisooctyl Polyhedral Oligo- SilSesquioxane (POSS) in polypropylene composites was evaluated in terms of thermal stability and fire retardancy and compared with talc. -

Evaluation of Selected Kaolin Clays As a Raw Material for the Turkish Cement and Concrete Industry
Evaluation of Selected Kaolin Clays as a Raw Material for the Turkish Cement and Concrete Industry Aydin Aras1, Mustafa Albayrak1, Metin Arikan2, Konstantin Sobolev3 * 1 General Directorate of Mineral Research and Exploration (MTA), Turkey 2 Civil Engineering Department, Middle East Technical University (METU), Turkey 3 Facultad de Ingenieria Civil, Universidad Autonoma de Nuevo Leon (UANL), Mexico ABSTRACT Turkey has a long tradition (starting from the prehistoric civilizations) and experience in exploring and processing clay raw materials into ceramic products. Many of these products, such as tiles and sanitary ware, are manufactured for domestic and export markets. Kaolin clay is one of the raw materials of major importance for the ceramic and paper industry, as well as for a number of auxiliary applications. There is an ongoing interest to apply kaolin clay in the construction industry as a raw material for the production of white cement clinker and as an artificial pozzolanic additive for concrete (in a form of metakaolin). This report presents the results related to search, assessment and evaluation of available resources for advanced cement and concrete additives. Keywords: kaolin, metakaolin, construction, resources, ceramics, cement, x-ray diffraction, SEM INTRODUCTION Turkey has an abundance of natural resources and its mining industry is one of the sectors showing steady growth. Among the most commonly mined minerals are borax, magnesite, chromites, barite, feldspars, different clays, and limestone [1-21]. Local ceramic industry has more than 4000 years of experience in exploring and processing widely available raw materials into useful commodities. Currently, several ceramic products, such as tiles and sanitary ware are manufactured to meet international standards (ISO 9000) and significant amounts (about 45 %) of these products are exported [21]. -

The Properties of Moulding Sand with Halloysite
ARCHIVES of ISSN (2299-2944) FOUNDRY ENGINEERING Volume 12 Issue 2/2012 DOI: 10.2478/v10266-012-0062-5 Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences 205 – 210 The Properties of Moulding Sand with Halloysite M. Cholewa, Ł. Kozakiewicz* Silesian University of Technology, Foundry Department, Towarowa 7, 44-100 Gliwice, PL *Corresponding author: E-mail address: [email protected] Received 25-05-2012; accepted in revised form 31-05-2012 Abstract Until now, the mould sand in general use in the foundry industry are based on bentonite, which resulted from the fact that a good recognition properties and phenomena associated with this material. Come to know and normalized content of montmorillonite and carbonates and their important role in the construction of bentonite, and mass properties of the participation of compressive strength or scatter. Halloysite is widely used in industry and beyond them. However, little is known about its use in the foundry in Poland and abroad. This article presents preliminary research conducted at the Foundry Department of Silesian University of Technology on this material. Will raise the question of the representation of this two materials, which contains information connected with history and formation of materials, their structure and chemical composition. In the research, the results of compressive strength tests in wet masses of quartz matrix, where as a binder is used halloysite and bentonite in different proportions. Keywords: Halloysite, Bentonite, Moulding Sand Automation in the previous period concerned the processes 1. Introduction related mainly to the implementation of form, hence the early arrangements are known as automatic molding lines (ALF). -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Kaolinite Al2si2o5(OH)4 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Triclinic
Kaolinite Al2Si2O5(OH)4 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Triclinic. Point Group: 1: Rarely as crystals, thin platy or stacked, to 2 mm. More commonly as microscopic pseudohexagonal plates and clusters of plates, aggregated into compact, claylike masses. Physical Properties: Cleavage: Perfect on 001 . Tenacity: Flexible but inelastic. Hardness = 2{2.5 D(meas.) = 2.61{2.68 D(caflc.) =g 2.63 Optical Properties: Transparent to translucent as single crystals. Color: White to tan, may be variously colored by impurities. Luster: Pearly to dull earthy. Optical Class: Biaxial ({). Orientation: X c = 13± to 10±; Y a = 1±{4±. Dispersion: r > v; weak. ® = 1.553{1.565^ ¯ =¡1.559{1¡.569 ° =^ 1.560{1.570 2V(meas.) = 24±{50± Cell Data: Space Group: P 1: a = 5.15 b = 8.95 c = 7.39 ® = 91:8± ¯ = 104:5± 105:0± ° = 90± Z = [2] ¡ X-ray Powder Pattern: Scalby, Yorkshire, England (1A). 7.16 (vvs), 3.573 (vvs), 4.336 (vs), 2.491 (s), 2.289 (s), 2.558 (ms), 2.379 (ms) Chemistry: (1) SiO2 45.80 Al2O3 39.55 Fe2O3 0.57 FeO 0.18 MgO 0.14 CaO 0.41 K2O 0.03 + H2O 13.92 H2O¡ 0.17 Total 100.77 3+ (1) Mikawo mine, Niigata Prefecture, Japan; corresponds to (Al2:00Fe0:02Mg0:01Ca0:02)§=2:05 Si2O5(OH)3:99: Polymorphism & Series: Dickite, halloysite, and nacrite are polymorphs. Mineral Group: Kaolinite-serpentine group. Occurrence: Replaces other aluminosilicate minerals during hydrothermal alteration and weathering. A common constituent of the clay-size fraction of sediments, where it may be formed by direct precipitation. -

Halloysite Nanotubes-Polymeric Nanocomposites: Characteristics, Modifications and Controlled Drug Delivery Approaches
423 Cerâmica 63 (2017) 423-431 http://dx.doi.org/10.1590/0366-69132017633682167 Halloysite nanotubes-polymeric nanocomposites: characteristics, modifications and controlled drug delivery approaches (Nanocompósitos poliméricos com nanotubos de haloisita: características, modificações e atuação como sistemas de liberação controlada de fármacos) P. C. Ferrari1, F. F. Araujo1, S. A. Pianaro2 1Department of Pharmaceutical Sciences; 2Department of Materials Engineering, State University of Ponta Grossa, Ponta Grossa, PR, Brazil Abstract Halloysite nanotubes (HNTs) are aluminosilicate nanoclay mineral which have a hollow tubular structure and occurs naturally. They are biocompatible and viable carrier for inclusion of biologically active molecules due to the empty space inside the tubular structure. In this article, the HNTs main characteristics, and the HNTs-polymeric nanocomposite formation and their potential application as improvement of the mechanical performance of polymers and entrapment of hydrophilic and lipophilic substances are summarized. Recent works covering the increment of HNTs-polymeric nanocomposites and presenting promising employment of these systems as nanosized carrier, being suitable for pharmaceutical and biomedical applications, based on earlier evidence in literature of its nature to sustain the release of loaded drugs, presenting low cytotoxicity, and providing evidence for controlled drug delivery are reviewed. Keywords: nanoclay, bio-nanocomposites, functionalization, sustained drug release, nanotechnology. Resumo Nanotubos de haloisita (NTH) são nanoargilas de silicato de alumínio com estrutura naturalmente tubular. Eles são biocompatíveis e apresentam viabilidade como carreador de moléculas biologicamente ativas devido ao seu espaço interno na estrutura tubular. Neste artigo as principais características dos NTH e a obtenção de nanocompósitos poliméricos com NTH, e suas potenciais aplicações para o aperfeiçoamento das propriedades mecânicas dos polímeros e como carreador de substâncias hidrofílicas e lipofílicas são revisadas. -

Halloysite Formation Through in Situ Weathering of Volcanic Glass From
Ciay Minerals (1988) 23, 423-431 mineralogy. Phys. ition of Mössbauer L HALLOYSITE FORMATION THROUGH Ih' SITU shaviour? J. Mag. WEATHERING OF VOLCANIC GLASS FROM 3n analysis of two TRACHYTIC PUMICES, VICO'S VOLCANO, ITALY d.X, 29-31. ir Conímbriga and P. QUANTIN, J. GAUTHEYROU AND P. LORENZONI* ts correlation with .central Portugal). ORSTOM, 70 route d'Aulnay. 93143 Bondy Cedex, France, and *ISSDS, Piazza d'Azeglio 30, Firenze, Italy ibrico da cerâmica (Received October 1987; revised 5 April 1988) of dolomites, clays ABSTRACT: The weathering of a trachytic pumice within a pyroclastic flow underlying an ., MADWCKA.G., andic-brown soil on the volcano Vico has been studied. The main mineral formed is a spherical 10 A halloysite which has been shown by SEM and in situ microprobe analysis to have formed norphology on the directly from the glass. The major mineralogical characteristics as determined by XRD, IR, DTA, TEM and microdiffraction are typical of 10 A halloysite. However, some minor mineralogical properties and the high Fe and K contents, suggest that it is an interstratification of 74% halloysite and 26% illite-smectite. The calculated formula of the hypothetical 2:l minerals reveals an Fe- and K-rich clay, with high tetrahedral substitution, like an Fe-rich vermiculite, but the detailed structure of this mineral remains uncertain. This study deals with the weathering of trachytic pumices to a white clay which seems to be derived directly from glass, without change in texture. This clay is a well crystallized 10 A halloysite, and although nearly white in colour, has an unusual composition being rich in Fe and Ti, and having a high K content. -

Eco-Friendly Betanin Hybrid Materials Based on Palygorskite and Halloysite
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 September 2020 Eco-friendly betanin hybrid materials based on palygorskite and halloysite Shue Li a,b,c, Bin Mu a,c*, Xiaowen Wang a,c, Yuru Kang a,c, Aiqin Wanga,c* a Key Laboratory of Clay Mineral Applied Research of Gansu Province, Center of Eco-Materials and Green Chemistry, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, P. R. China b Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing, P. R. China c Center of Xuyi Palygorskite Applied Technology, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Xuyi, P. R. China ABSTRAC Eco-friendly betanin/clay minerals hybrid materials with good stability were synthesized combining natural betanin molecules extracted from beetroot with 2:1 type palygorskite (Pal) and 1:1 type halloysite (Hal), respectively. It was found that the adsorption, grinding and heating treatment played a key role to enhance the interaction between betanin and clay minerals during preparation process, which favored improving the thermal stability and solvent resistance of natural betanin. The L* and a* values of the betanin/Pal and betanin/Hal hybrid materials were 64.94 and 14.96, 62.55 and 15.48, respectively, indicating that betanin/Hal exhibited the better color performance. The structural characterizations *Corresponding authors. E-mail addresses: [email protected] (B. Mu) and [email protected] (A. Wang); Fax: +86 931 4968019; Tel: +86 931 4868118. 1 © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 September 2020 confirmed that betanin was mainly adsorbed on the outer surface of Pal or Hal through hydrogen-bond interaction, and part of them also were entered into the inner surface of Hal via electrostatic interaction. -

Effect of Kaolinite and Ca-Montmorillonite on the Alleviation of Soil Water Repellency
Effect of kaolinite and Ca-montmorillonite on the alleviation of soil water repellency P. Dlapa1, S.H. Doerr2, Ľ. Lichner3, M. Šír4, M. Tesař4 1Faculty of Natural Science, Comenius University, Bratislava, Slovakia 2Department of Geography, University of Wales Swansea, Swansea, UK 3Institute of Hydrology, Slovak Academy of Sciences, Bratislava, Slovakia 4Institute for Hydrodynamics, Academy of Sciences of the Czech Republic, Prague, Czech Republic ABSTRACT The effects of adding 1–3% (weight) kaolinite or Ca-montmorillonite on the we�ability of silica sand, made highly water repellent with stearic acid, was studied during we�ing and prolonged drying phases at 50°C. The persistence of water repellency was estimated with the water drop penetration time (WDPT) test. A�er we�ing water repellency disappeared in all the samples. During the drying phase, water repellency re-appeared in all samples (untreated and clay-treated) as the water content decreased below 1%. Repellency did, however, not reach pre-we�ing levels. The effect of clay additions on water repellency differed strongly between the two clay types. Kaolinite reduced WDPT, while Ca-montmorillonite caused an increase in WDPT in the already highly repellent sand. Potential mechanisms for the alleviation effectiveness of kaolinite are proposed, with key factors being the high adhesion forces between water and clay mineral surfaces, and the ability kaolinite to disperse. In the case of Ca-montmorillonite, its lower affinity for water may lead to a displacement of water molecules at mineral surfaces by amphiphilic organic compounds, which may result in increased repellency. This phenomenon clearly requires further investigation. Keywords: water repellency; kaolinite; Ca-montmorillonite; stearic acid The occurrence of water repellency in many soils to wind erosion. -
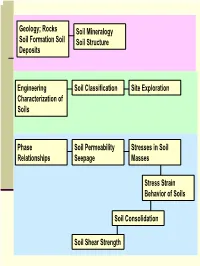
Rocks Soil Formation Soil Deposits Soil Mineralogy Soil Structure
Geology; Rocks Soil Mineralogy Soil Formation Soil Soil Structure Deposits Engineering Soil Classification Site Exploration Characterization of Soils Phase Soil Permeability Stresses in Soil Relationships Seepage Masses Stress Strain Behavior of Soils Soil Consolidation Soil Shear Strength 1 Engineering Characterization of Soils Soil Properties that Control its Engineering Behavior Particle Size − Sieve Analysis − Hydrometer Analysis coarse-grained fine-grained Particle/Grain Size Soil Plasticity Distribution Particle Shapes (?) 2 Particle Size; Standard Sieve Sizes 3 ASTM Particle Size Classification 4 Sieve Analysis (Mechanical Analysis) This procedure is suitable for coarse grained soils See next slide for ASTM Standard Sieves No.10 sieve …. Has 10 apertures per linear inch 5 ASTM Standard Sieves 6 Hydrometer Analysis Also called Sedimentation Analysis Stoke’s Law D2γ (G − G ) v = w s L 18η 7 Grain Size Distribution Curves 8 Terminology C….. Poorly-graded soil D …. Well-graded soil E …. Gap-graded soil D10, D30, D60 = ?? Coefficient of Uniformity, Cu= D60/D10 Coefficient of Curvature, )2 Cc= (D30 /(D10)(D60) 9 Particle Distribution Calculations Example 10 Particle Shapes 11 Clay Formation Clay particles < 2 µm Compared to Sands and Silts, clay size particles have undergone a lot more “chemical weathering”! 12 Clay vs. Sand/Silt Clay particles are generally more platy in shape (sand more equi-dimensional) Clay particles carry surface charge Amount of surface charge depends on type of clay minerals Surface charges that -

Phase Transformations in Silica-Alumina- Magnesia Mixtures As Examined by Continuous X-Ray Diffraction: I
THE AMERICAN MINERALOGIST, VOL. 47, NOVEMBER-DECEMBER, 1962 PHASE TRANSFORMATIONS IN SILICA-ALUMINA- MAGNESIA MIXTURES AS EXAMINED BY CONTINUOUS X-RAY DIFFRACTION: I. TALC-KAOLINITE COMPOSITIONS R. B. Gnen,lF. M. Wnnr, aNp R. E. Gnru, Uniaersityof Illinois, (Jrbana, I llinois. Ansmacr The phase transformations of natural and synthetic oxide mixtures of talc-kaolinite compositlons were examined by continuous r-ray difiraction while heated to 1450' C. Dif- fraciion intensity data show the enhanced development of protoenstatite within the sheet structure of talc, thus indicating that the growth of this mineral on firing is largely con- trolled by the structure of the talc. The formation temperatule of cordierite, which was used as a measure of the reactivity of various starting materials, indicates that alumina in the form of mullite, rather than as corundum, is most efiective in lowering the develop- ment temperature of this mineral. Additional cordierite formation, through recrystalliza- tion on cooling indicates the presence of an intermediate licluid phase in the formation of this mineral. INrnotucrlow Generalsta,tement . Continuous high-temperature o-ray diffraction methods make it pos- sible to observehigh-temperature reactions as they occur (Wahl et aI., 1961). Previously, high-temperatrte n-ray diffraction has been used mainly for selected observations at some desired temperature and not for continuousobservations of a sampleas it is heated.The techniqueof continuous scanning was used in the present investigation for the study of high-temperaturereactions in talc, kaolinite, talc:kaolinite mixtures, and mixtures of oxides with compositions corresponding to talc and kaolinite. Infl,uence of starting materials Reactionrates between solids are known to be influencedby the nature of the starting materials. -

Applications of Halloysite Nanocontainers for Functional Protective Coating Anupam Ramesh Joshi
Louisiana Tech University Louisiana Tech Digital Commons Doctoral Dissertations Graduate School Fall 2014 Applications of halloysite nanocontainers for functional protective coating Anupam Ramesh Joshi Follow this and additional works at: https://digitalcommons.latech.edu/dissertations Part of the Nanoscience and Nanotechnology Commons APPLICATIONS OF HALLOYSITE NANOCONTAINERS FOR FUNCTIONAL PROTECTIVE COATING by, Anupam Ramesh Joshi, B.Sc., M.Sc., M.S. A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy COLLEGE OF ENGINEERING AND SCIENCE LOUISIANA TECH UNIVERSITY November 2014 UMI Number: 3662476 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Di!ss0?t&Ciori P iib list’Mlg UMI 3662476 Published by ProQuest LLC 2015. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 LOUISIANA TECH UNIVERSITY THE GRADUATE SCHOOL ________ NOVEMBER 15, 2014 Date We hereby recommend that the dissertation prepared under our supervision by ANUPAM RAMESH JOSHI, B.Sc., M.Sc., M.S.__________________________________