Preparation and Characterization of Kaolinite Nanostructures: Reaction Pathways, Morphology and Structural Order
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fire Retardancy of Polypropylene/Kaolinite Composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta
Fire retardancy of polypropylene/kaolinite composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta To cite this version: Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta. Fire retardancy of polypropylene/kaolinite composites. Polymer Degradation and Stability, Elsevier, 2016, 129, pp.260-267. 10.1016/j.polymdegradstab.2016.05.003. hal-02906432 HAL Id: hal-02906432 https://hal.archives-ouvertes.fr/hal-02906432 Submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Fire retardancy of polypropylene/kaolinite composites * Marcos Batistella a, c, , Belkacem Otazaghine b, Rodolphe Sonnier b, Carlos Petter c, Jose-Marie Lopez-Cuesta b a Federal University of Santa Catarina, R. Eng. Agronomico^ Andrei Cristian Ferreira, s/n e Trindade, Florianopolis, SC, CEP 88040-900, Brazil b Ecole des Mines d’Ales, Centre des Materiaux (C2MA) e Pole^ Materiaux Polymeres Avances, 6 Avenue de Clavieres, 30319, Ales Cedex, France c Federal University of Rio Grande do Sul, Av. Bento Gonçalves, 9500, Porto Alegre, CEP 91501-970, Brazil abstract In this study the influence of surface modification of kaolinite with trisilanolisooctyl Polyhedral Oligo- SilSesquioxane (POSS) in polypropylene composites was evaluated in terms of thermal stability and fire retardancy and compared with talc. -

Evaluation of Selected Kaolin Clays As a Raw Material for the Turkish Cement and Concrete Industry
Evaluation of Selected Kaolin Clays as a Raw Material for the Turkish Cement and Concrete Industry Aydin Aras1, Mustafa Albayrak1, Metin Arikan2, Konstantin Sobolev3 * 1 General Directorate of Mineral Research and Exploration (MTA), Turkey 2 Civil Engineering Department, Middle East Technical University (METU), Turkey 3 Facultad de Ingenieria Civil, Universidad Autonoma de Nuevo Leon (UANL), Mexico ABSTRACT Turkey has a long tradition (starting from the prehistoric civilizations) and experience in exploring and processing clay raw materials into ceramic products. Many of these products, such as tiles and sanitary ware, are manufactured for domestic and export markets. Kaolin clay is one of the raw materials of major importance for the ceramic and paper industry, as well as for a number of auxiliary applications. There is an ongoing interest to apply kaolin clay in the construction industry as a raw material for the production of white cement clinker and as an artificial pozzolanic additive for concrete (in a form of metakaolin). This report presents the results related to search, assessment and evaluation of available resources for advanced cement and concrete additives. Keywords: kaolin, metakaolin, construction, resources, ceramics, cement, x-ray diffraction, SEM INTRODUCTION Turkey has an abundance of natural resources and its mining industry is one of the sectors showing steady growth. Among the most commonly mined minerals are borax, magnesite, chromites, barite, feldspars, different clays, and limestone [1-21]. Local ceramic industry has more than 4000 years of experience in exploring and processing widely available raw materials into useful commodities. Currently, several ceramic products, such as tiles and sanitary ware are manufactured to meet international standards (ISO 9000) and significant amounts (about 45 %) of these products are exported [21]. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Kaolinite Al2si2o5(OH)4 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Triclinic
Kaolinite Al2Si2O5(OH)4 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Triclinic. Point Group: 1: Rarely as crystals, thin platy or stacked, to 2 mm. More commonly as microscopic pseudohexagonal plates and clusters of plates, aggregated into compact, claylike masses. Physical Properties: Cleavage: Perfect on 001 . Tenacity: Flexible but inelastic. Hardness = 2{2.5 D(meas.) = 2.61{2.68 D(caflc.) =g 2.63 Optical Properties: Transparent to translucent as single crystals. Color: White to tan, may be variously colored by impurities. Luster: Pearly to dull earthy. Optical Class: Biaxial ({). Orientation: X c = 13± to 10±; Y a = 1±{4±. Dispersion: r > v; weak. ® = 1.553{1.565^ ¯ =¡1.559{1¡.569 ° =^ 1.560{1.570 2V(meas.) = 24±{50± Cell Data: Space Group: P 1: a = 5.15 b = 8.95 c = 7.39 ® = 91:8± ¯ = 104:5± 105:0± ° = 90± Z = [2] ¡ X-ray Powder Pattern: Scalby, Yorkshire, England (1A). 7.16 (vvs), 3.573 (vvs), 4.336 (vs), 2.491 (s), 2.289 (s), 2.558 (ms), 2.379 (ms) Chemistry: (1) SiO2 45.80 Al2O3 39.55 Fe2O3 0.57 FeO 0.18 MgO 0.14 CaO 0.41 K2O 0.03 + H2O 13.92 H2O¡ 0.17 Total 100.77 3+ (1) Mikawo mine, Niigata Prefecture, Japan; corresponds to (Al2:00Fe0:02Mg0:01Ca0:02)§=2:05 Si2O5(OH)3:99: Polymorphism & Series: Dickite, halloysite, and nacrite are polymorphs. Mineral Group: Kaolinite-serpentine group. Occurrence: Replaces other aluminosilicate minerals during hydrothermal alteration and weathering. A common constituent of the clay-size fraction of sediments, where it may be formed by direct precipitation. -

The Influence of Halloysite Content on the Shear Strength of Kaolinite
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1981 The influence of halloysite content on the shear strength of kaolinite Reka Katalin Gabor Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Geology Commons, and the Materials Science and Engineering Commons Let us know how access to this document benefits ou.y Recommended Citation Gabor, Reka Katalin, "The influence of halloysite content on the shear strength of kaolinite" (1981). Dissertations and Theses. Paper 3215. https://doi.org/10.15760/etd.3206 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF THE THESIS OF Reka Katalin Gabor for the Master of Science in Geology presented October 6, 1981. Title: The Influence of Halloysite Content on the Shear Strength of Kaolinite. APPROVED BY MEMBERS OF THE THESIS COMMITTEE: The objective of this thesis is to determine the rel ative shear strengths of halloysite, kaolinite, synthetic mixtures, and local soils, to investigate the influence of halloysite content on the shear strength of kaolinite, and to explore the possibility that the strength properties of soil clays might be controlled by the relative content of their component minerals. Sets of samples of pure kaolinite and halloysite min erals and their mixtures in proportions of 1:1, 3:1, and 2 1:3 were prepared in the Harvard Miniature Compaction de vice, each compacted in four separate layers with 35 tamp- ings from the 30 pound spring compactor on each layer. -

Effect of Kaolinite and Ca-Montmorillonite on the Alleviation of Soil Water Repellency
Effect of kaolinite and Ca-montmorillonite on the alleviation of soil water repellency P. Dlapa1, S.H. Doerr2, Ľ. Lichner3, M. Šír4, M. Tesař4 1Faculty of Natural Science, Comenius University, Bratislava, Slovakia 2Department of Geography, University of Wales Swansea, Swansea, UK 3Institute of Hydrology, Slovak Academy of Sciences, Bratislava, Slovakia 4Institute for Hydrodynamics, Academy of Sciences of the Czech Republic, Prague, Czech Republic ABSTRACT The effects of adding 1–3% (weight) kaolinite or Ca-montmorillonite on the we�ability of silica sand, made highly water repellent with stearic acid, was studied during we�ing and prolonged drying phases at 50°C. The persistence of water repellency was estimated with the water drop penetration time (WDPT) test. A�er we�ing water repellency disappeared in all the samples. During the drying phase, water repellency re-appeared in all samples (untreated and clay-treated) as the water content decreased below 1%. Repellency did, however, not reach pre-we�ing levels. The effect of clay additions on water repellency differed strongly between the two clay types. Kaolinite reduced WDPT, while Ca-montmorillonite caused an increase in WDPT in the already highly repellent sand. Potential mechanisms for the alleviation effectiveness of kaolinite are proposed, with key factors being the high adhesion forces between water and clay mineral surfaces, and the ability kaolinite to disperse. In the case of Ca-montmorillonite, its lower affinity for water may lead to a displacement of water molecules at mineral surfaces by amphiphilic organic compounds, which may result in increased repellency. This phenomenon clearly requires further investigation. Keywords: water repellency; kaolinite; Ca-montmorillonite; stearic acid The occurrence of water repellency in many soils to wind erosion. -
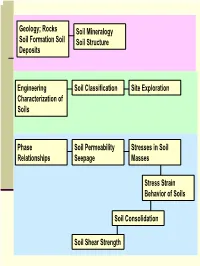
Rocks Soil Formation Soil Deposits Soil Mineralogy Soil Structure
Geology; Rocks Soil Mineralogy Soil Formation Soil Soil Structure Deposits Engineering Soil Classification Site Exploration Characterization of Soils Phase Soil Permeability Stresses in Soil Relationships Seepage Masses Stress Strain Behavior of Soils Soil Consolidation Soil Shear Strength 1 Engineering Characterization of Soils Soil Properties that Control its Engineering Behavior Particle Size − Sieve Analysis − Hydrometer Analysis coarse-grained fine-grained Particle/Grain Size Soil Plasticity Distribution Particle Shapes (?) 2 Particle Size; Standard Sieve Sizes 3 ASTM Particle Size Classification 4 Sieve Analysis (Mechanical Analysis) This procedure is suitable for coarse grained soils See next slide for ASTM Standard Sieves No.10 sieve …. Has 10 apertures per linear inch 5 ASTM Standard Sieves 6 Hydrometer Analysis Also called Sedimentation Analysis Stoke’s Law D2γ (G − G ) v = w s L 18η 7 Grain Size Distribution Curves 8 Terminology C….. Poorly-graded soil D …. Well-graded soil E …. Gap-graded soil D10, D30, D60 = ?? Coefficient of Uniformity, Cu= D60/D10 Coefficient of Curvature, )2 Cc= (D30 /(D10)(D60) 9 Particle Distribution Calculations Example 10 Particle Shapes 11 Clay Formation Clay particles < 2 µm Compared to Sands and Silts, clay size particles have undergone a lot more “chemical weathering”! 12 Clay vs. Sand/Silt Clay particles are generally more platy in shape (sand more equi-dimensional) Clay particles carry surface charge Amount of surface charge depends on type of clay minerals Surface charges that -

Phase Transformations in Silica-Alumina- Magnesia Mixtures As Examined by Continuous X-Ray Diffraction: I
THE AMERICAN MINERALOGIST, VOL. 47, NOVEMBER-DECEMBER, 1962 PHASE TRANSFORMATIONS IN SILICA-ALUMINA- MAGNESIA MIXTURES AS EXAMINED BY CONTINUOUS X-RAY DIFFRACTION: I. TALC-KAOLINITE COMPOSITIONS R. B. Gnen,lF. M. Wnnr, aNp R. E. Gnru, Uniaersityof Illinois, (Jrbana, I llinois. Ansmacr The phase transformations of natural and synthetic oxide mixtures of talc-kaolinite compositlons were examined by continuous r-ray difiraction while heated to 1450' C. Dif- fraciion intensity data show the enhanced development of protoenstatite within the sheet structure of talc, thus indicating that the growth of this mineral on firing is largely con- trolled by the structure of the talc. The formation temperatule of cordierite, which was used as a measure of the reactivity of various starting materials, indicates that alumina in the form of mullite, rather than as corundum, is most efiective in lowering the develop- ment temperature of this mineral. Additional cordierite formation, through recrystalliza- tion on cooling indicates the presence of an intermediate licluid phase in the formation of this mineral. INrnotucrlow Generalsta,tement . Continuous high-temperature o-ray diffraction methods make it pos- sible to observehigh-temperature reactions as they occur (Wahl et aI., 1961). Previously, high-temperatrte n-ray diffraction has been used mainly for selected observations at some desired temperature and not for continuousobservations of a sampleas it is heated.The techniqueof continuous scanning was used in the present investigation for the study of high-temperaturereactions in talc, kaolinite, talc:kaolinite mixtures, and mixtures of oxides with compositions corresponding to talc and kaolinite. Infl,uence of starting materials Reactionrates between solids are known to be influencedby the nature of the starting materials. -

Mineralogical Studies of Kaolinite-Halloysite Clays: Part Iii
THE AMERICAN MINERALOGIST, VOL. 50, MAY_JUNE, 1965 MINERALOGICAL STUDIES OF KAOLINITE-HALLOYSITE CLAYS: PART III. A FIBROUS KAOLIN MINERAL FROM PIEDADE, SAO PAULO, BRAZIL PE,nsroDE SouzA SeNros, Instituto ile PesquisasTecnologicas, University of Sd.oPaulo, Sdo Paulo, Brazil, G. W. Bnrnlr.nv, Departmentof Geochemistryand. Minerology, The Pennsylaani,a Slate (Jniaers'ity,Uniaersity Park, Po., aNn HBTBNI oB Souze SaNros, Centro de Microscopia Electronico, (Jn'iversityof Sd,oPaulo. Sao Paulo. Brazil. Arsrn,rcr An unusual form of kaolin mineral, filling cracks in weathered prophyritic granite in the countl, of Piedade, Sdo Paulo, Brazil, consists of visibly fibrous material which, in the electron microscope, appears as thin tubular fibers of considerable length; platy particles are almost entirely absent. X-ray rotation diagrams of dried fiber bundles and selected area electron diffraction patterns of single fibers show an appreciable degree of structural regu- larity in the kaolin layer structure, with 6 as the fiber axis. The results show generar agree- ment with previous data by Honjo, Kitamura and Mihama. Prior to drying, the material as collected can be described as consisting of f endellite and $ halloysite. The endellite com- ponent has the swelling and shrinking behavior characteristic of this mineral with respect to ethylene glycol, water, and gentle heat treatments. This may be the first observation of a mineral of endellite-halloysite type existing in a directly visible fibrous form. INrnolucrror.l An unusual form of kaolin clay -

Clay Minerals
CLAY MINERALS CD. Barton United States Department of Agriculture Forest Service, Aiken, South Carolina, U.S.A. A.D. Karathanasis University of Kentucky, Lexington, Kentucky, U.S.A. INTRODUCTION of soil minerals is understandable. Notwithstanding, the prevalence of silicon and oxygen in the phyllosilicate structure is logical. The SiC>4 tetrahedron is the foundation Clay minerals refers to a group of hydrous aluminosili- 2 of all silicate structures. It consists of four O ~~ ions at the cates that predominate the clay-sized (<2 |xm) fraction of apices of a regular tetrahedron coordinated to one Si4+ at soils. These minerals are similar in chemical and structural the center (Fig. 1). An interlocking array of these composition to the primary minerals that originate from tetrahedral connected at three corners in the same plane the Earth's crust; however, transformations in the by shared oxygen anions forms a hexagonal network geometric arrangement of atoms and ions within their called the tetrahedral sheet (2). When external ions bond to structures occur due to weathering. Primary minerals form the tetrahedral sheet they are coordinated to one hydroxyl at elevated temperatures and pressures, and are usually and two oxygen anion groups. An aluminum, magnesium, derived from igneous or metamorphic rocks. Inside the or iron ion typically serves as the coordinating cation and Earth these minerals are relatively stable, but transform- is surrounded by six oxygen atoms or hydroxyl groups ations may occur once exposed to the ambient conditions resulting in an eight-sided building block termed an of the Earth's surface. Although some of the most resistant octohedron (Fig. -

Glossary of Geological Terms
GLOSSARY OF GEOLOGICAL TERMS These terms relate to prospecting and exploration, to the regional geology of Newfoundland and Labrador, and to some of the geological environments and mineral occurrences preserved in the province. Some common rocks, textures and structural terms are also defined. You may come across some of these terms when reading company assessment files, government reports or papers from journals. Underlined words in definitions are explained elsewhere in the glossary. New material will be added as needed - check back often. - A - A-HORIZON SOIL: the uppermost layer of soil also referred to as topsoil. This is the layer of mineral soil with the most organic matter accumulation and soil life. This layer is not usually selected in soil surveys. ADIT: an opening that is driven horizontally (into the side of a mountain or hill) to access a mineral deposit. AIRBORNE SURVEY: a geophysical survey done from the air by systematically crossing an area or mineral property using aircraft outfitted with a variety of sensitive instruments designed to measure variations in the earth=s magnetic, gravitational, electro-magnetic fields, and/or the radiation (Radiometric Surveys) emitted by rocks at or near the surface. These surveys detect anomalies. AIRBORNE MAGNETIC (or AEROMAG) SURVEYS: regional or local magnetic surveys that measures deviations in the earth=s magnetic field and carried out by flying a magnetometer along flight lines on a pre-determined grid pattern. The lower the aircraft and the closer the flight lines, the more sensitive is the survey and the more detail in the resultant maps. Aeromag maps produced from these surveys are important exploration tools and have played a major role in many major discoveries (e.g., the Olympic Dam deposit in Australia). -

A Partial Glossary of Spanish Geological Terms Exclusive of Most Cognates
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY A Partial Glossary of Spanish Geological Terms Exclusive of Most Cognates by Keith R. Long Open-File Report 91-0579 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. 1991 Preface In recent years, almost all countries in Latin America have adopted democratic political systems and liberal economic policies. The resulting favorable investment climate has spurred a new wave of North American investment in Latin American mineral resources and has improved cooperation between geoscience organizations on both continents. The U.S. Geological Survey (USGS) has responded to the new situation through cooperative mineral resource investigations with a number of countries in Latin America. These activities are now being coordinated by the USGS's Center for Inter-American Mineral Resource Investigations (CIMRI), recently established in Tucson, Arizona. In the course of CIMRI's work, we have found a need for a compilation of Spanish geological and mining terminology that goes beyond the few Spanish-English geological dictionaries available. Even geologists who are fluent in Spanish often encounter local terminology oijerga that is unfamiliar. These terms, which have grown out of five centuries of mining tradition in Latin America, and frequently draw on native languages, usually cannot be found in standard dictionaries. There are, of course, many geological terms which can be recognized even by geologists who speak little or no Spanish.