Phylogenysupm Vitales2017.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014
The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014 In the pages that follow are treatments that have been revised since the publication of the Jepson eFlora, Revision 1 (July 2013). The information in these revisions is intended to supersede that in the second edition of The Jepson Manual (2012). The revised treatments, as well as errata and other small changes not noted here, are included in the Jepson eFlora (http://ucjeps.berkeley.edu/IJM.html). For a list of errata and small changes in treatments that are not included here, please see: http://ucjeps.berkeley.edu/JM12_errata.html Citation for the entire Jepson eFlora: Jepson Flora Project (eds.) [year] Jepson eFlora, http://ucjeps.berkeley.edu/IJM.html [accessed on month, day, year] Citation for an individual treatment in this supplement: [Author of taxon treatment] 2014. [Taxon name], Revision 2, in Jepson Flora Project (eds.) Jepson eFlora, [URL for treatment]. Accessed on [month, day, year]. Copyright © 2014 Regents of the University of California Supplement II, Page 1 Summary of changes made in Revision 2 of the Jepson eFlora, December 2014 PTERIDACEAE *Pteridaceae key to genera: All of the CA members of Cheilanthes transferred to Myriopteris *Cheilanthes: Cheilanthes clevelandii D. C. Eaton changed to Myriopteris clevelandii (D. C. Eaton) Grusz & Windham, as native Cheilanthes cooperae D. C. Eaton changed to Myriopteris cooperae (D. C. Eaton) Grusz & Windham, as native Cheilanthes covillei Maxon changed to Myriopteris covillei (Maxon) Á. Löve & D. Löve, as native Cheilanthes feei T. Moore changed to Myriopteris gracilis Fée, as native Cheilanthes gracillima D. -

Molecular Phylogeny of Subtribe Artemisiinae (Asteraceae), Including Artemisia and Its Allied and Segregate Genera Linda E
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications in the Biological Sciences Papers in the Biological Sciences 9-26-2002 Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera Linda E. Watson Miami University, [email protected] Paul E. Bates University of Nebraska-Lincoln, [email protected] Timonthy M. Evans Hope College, [email protected] Matthew M. Unwin Miami University, [email protected] James R. Estes University of Nebraska State Museum, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/bioscifacpub Watson, Linda E.; Bates, Paul E.; Evans, Timonthy M.; Unwin, Matthew M.; and Estes, James R., "Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera" (2002). Faculty Publications in the Biological Sciences. 378. http://digitalcommons.unl.edu/bioscifacpub/378 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications in the Biological Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. BMC Evolutionary Biology BioMed Central Research2 BMC2002, Evolutionary article Biology x Open Access Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera Linda E Watson*1, Paul L Bates2, Timothy M Evans3, -

Types of Sagebrush Updated (Artemisia Subg. Tridentatae
Mosyakin, S.L., L.M. Shultz & G.V. Boiko. 2017. Types of sagebrush updated ( Artemisia subg. Tridentatae, Asteraceae): miscellaneous comments and additional specimens from the Besser and Turczaninov memorial herbaria (KW). Phytoneuron 2017-25: 1–20. Published 6 April 2017. ISSN 2153 733X TYPES OF SAGEBRUSH UPDATED (ARTEMISIA SUBG. TRIDENTATAE , ASTERACEAE): MISCELLANEOUS COMMENTS AND ADDITIONAL SPECIMENS FROM THE BESSER AND TURCZANINOV MEMORIAL HERBARIA (KW) SERGEI L. MOSYAKIN M.G. Kholodny Institute of Botany National Academy of Sciences of Ukraine 2 Tereshchenkivska Street Kiev (Kyiv), 01004 Ukraine [email protected] LEILA M. SHULTZ Department of Wildland Resources, NR 329 Utah State University Logan, Utah 84322-5230, USA [email protected] GANNA V. BOIKO M.G. Kholodny Institute of Botany National Academy of Sciences of Ukraine 2 Tereshchenkivska Street Kiev (Kyiv), 01004 Ukraine [email protected] ABSTRACT Corrections and additions are provided for the existing typifications of plant names in Artemisia subg. Tridentatae . In particular, second-step lectotypifications are proposed for the names Artemisia trifida Nutt., nom. illeg. (A. tripartita Rydb., the currently accepted replacement name), A. fischeriana Besser (= A. californica Lessing, the currently accepted name), and A. pedatifida Nutt. For several nomenclatural types of names listed in earlier publications as "holotypes," the type designations are corrected to lectotypes (Art. 9.9. of ICN ). Newly discovered authentic specimens (mostly isolectotypes) of several names in the group are listed and discussed, mainly based on specimens deposited in the Besser and Turczaninov memorial herbaria at the National Herbarium of Ukraine (KW). The Turczaninov herbarium is particularly rich in Nuttall's specimens, which are often better represented and better preserved than corresponding specimens available from BM, GH, K, PH, and some other major herbaria. -

Anthemideae Christoph Oberprieler, Sven Himmelreich, Mari Källersjö, Joan Vallès, Linda E
Chapter38 Anthemideae Christoph Oberprieler, Sven Himmelreich, Mari Källersjö, Joan Vallès, Linda E. Watson and Robert Vogt HISTORICAL OVERVIEW The circumscription of Anthemideae remained relatively unchanged since the early artifi cial classifi cation systems According to the most recent generic conspectus of Com- of Lessing (1832), Hoff mann (1890–1894), and Bentham pos itae tribe Anthemideae (Oberprieler et al. 2007a), the (1873), and also in more recent ones (e.g., Reitbrecht 1974; tribe consists of 111 genera and ca. 1800 species. The Heywood and Humphries 1977; Bremer and Humphries main concentrations of members of Anthemideae are in 1993), with Cotula and Ursinia being included in the tribe Central Asia, the Mediterranean region, and southern despite extensive debate (Bentham 1873; Robinson and Africa. Members of the tribe are well known as aromatic Brettell 1973; Heywood and Humphries 1977; Jeff rey plants, and some are utilized for their pharmaceutical 1978; Gadek et al. 1989; Bruhl and Quinn 1990, 1991; and/or pesticidal value (Fig. 38.1). Bremer and Humphries 1993; Kim and Jansen 1995). The tribe Anthemideae was fi rst described by Cassini Subtribal classifi cation, however, has created considerable (1819: 192) as his eleventh tribe of Compositae. In a diffi culties throughout the taxonomic history of the tribe. later publication (Cassini 1823) he divided the tribe into Owing to the artifi ciality of a subtribal classifi cation based two major groups: “Anthémidées-Chrysanthémées” and on the presence vs. absence of paleae, numerous attempts “An thé midées-Prototypes”, based on the absence vs. have been made to develop a more satisfactory taxonomy presence of paleae (receptacular scales). -

Molecular Phylogeny of Chrysanthemum , Ajania and Its Allies (Anthemideae, Asteraceae) As Inferred from Nuclear Ribosomal ITS and Chloroplast Trn LF IGS Sequences
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/248021556 Molecular phylogeny of Chrysanthemum , Ajania and its allies (Anthemideae, Asteraceae) as inferred from nuclear ribosomal ITS and chloroplast trn LF IGS sequences ARTICLE in PLANT SYSTEMATICS AND EVOLUTION · FEBRUARY 2010 Impact Factor: 1.42 · DOI: 10.1007/s00606-009-0242-0 CITATIONS READS 25 117 5 AUTHORS, INCLUDING: Hongbo Zhao Sumei Chen Zhejiang A&F University Nanjing Agricultural University 15 PUBLICATIONS 56 CITATIONS 97 PUBLICATIONS 829 CITATIONS SEE PROFILE SEE PROFILE All in-text references underlined in blue are linked to publications on ResearchGate, Available from: Hongbo Zhao letting you access and read them immediately. Retrieved on: 02 December 2015 Plant Syst Evol (2010) 284:153–169 DOI 10.1007/s00606-009-0242-0 ORIGINAL ARTICLE Molecular phylogeny of Chrysanthemum, Ajania and its allies (Anthemideae, Asteraceae) as inferred from nuclear ribosomal ITS and chloroplast trnL-F IGS sequences Hong-Bo Zhao • Fa-Di Chen • Su-Mei Chen • Guo-Sheng Wu • Wei-Ming Guo Received: 14 April 2009 / Accepted: 25 October 2009 / Published online: 4 December 2009 Ó Springer-Verlag 2009 Abstract To better understand the evolutionary history, positions of some ambiguous taxa were renewedly con- intergeneric relationships and circumscription of Chry- sidered. Subtribe Artemisiinae was chiefly divided into two santhemum and Ajania and the taxonomic position of groups, (1) one corresponding to Chrysanthemum, Arc- some small Asian genera (Anthemideae, Asteraceae), the tanthemum, Ajania, Opisthopappus and Elachanthemum sequences of the nuclear ribosomal internal transcribed (the Chrysanthemum group), (2) another to Artemisia, spacer (nrDNA ITS) and the chloroplast trnL-F intergenic Crossostephium, Neopallasia and Sphaeromeria (the spacer (cpDNA IGS) were newly obtained for 48 taxa and Artemisia group). -

Washington Flora Checklist a Checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium
Washington Flora Checklist A checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium The Washington Flora Checklist aims to be a complete list of the native and naturalized vascular plants of Washington State, with current classifications, nomenclature and synonymy. The checklist currently contains 3,929 terminal taxa (species, subspecies, and varieties). Taxa included in the checklist: * Native taxa whether extant, extirpated, or extinct. * Exotic taxa that are naturalized, escaped from cultivation, or persisting wild. * Waifs (e.g., ballast plants, escaped crop plants) and other scarcely collected exotics. * Interspecific hybrids that are frequent or self-maintaining. * Some unnamed taxa in the process of being described. Family classifications follow APG IV for angiosperms, PPG I (J. Syst. Evol. 54:563?603. 2016.) for pteridophytes, and Christenhusz et al. (Phytotaxa 19:55?70. 2011.) for gymnosperms, with a few exceptions. Nomenclature and synonymy at the rank of genus and below follows the 2nd Edition of the Flora of the Pacific Northwest except where superceded by new information. Accepted names are indicated with blue font; synonyms with black font. Native species and infraspecies are marked with boldface font. Please note: This is a working checklist, continuously updated. Use it at your discretion. Created from the Washington Flora Checklist Database on September 17th, 2018 at 9:47pm PST. Available online at http://biology.burke.washington.edu/waflora/checklist.php Comments and questions should be addressed to the checklist administrators: David Giblin ([email protected]) Peter Zika ([email protected]) Suggested citation: Weinmann, F., P.F. Zika, D.E. Giblin, B. -

Bulletin of the Natural History Museum
Bulletin of _ The Natural History Bfit-RSH MU8&M PRIteifTBD QENERAl LIBRARY Botany Series VOLUME 23 NUMBER 2 25 NOVEMBER 1993 The Bulletin of The Natural History Museum (formerly: Bulletin of the British Museum (Natural History)), instituted in 1949, is issued in four scientific series, Botany, Entomology, Geology (incorporating Mineralogy) and Zoology. The Botany Series is edited in the Museum's Department of Botany Keeper of Botany: Dr S. Blackmore Editor of Bulletin: Dr R. Huxley Assistant Editor: Mrs M.J. West Papers in the Bulletin are primarily the results of research carried out on the unique and ever- growing collections of the Museum, both by the scientific staff and by specialists from elsewhere who make use of the Museum's resources. Many of the papers are works of reference that will remain indispensable for years to come. All papers submitted for publication are subjected to external peer review for acceptance. A volume contains about 160 pages, made up by two numbers, published in the Spring and Autumn. Subscriptions may be placed for one or more of the series on an annual basis. Individual numbers and back numbers can be purchased and a Bulletin catalogue, by series, is available. Orders and enquiries should be sent to: Intercept Ltd. P.O. Box 716 Andover Hampshire SPIO lYG Telephone: (0264) 334748 Fax: (0264) 334058 WorW Lwr abbreviation: Bull. nat. Hist. Mus. Lond. (Bot.) © The Natural History Museum, 1993 Botany Series ISSN 0968-0446 Vol. 23, No. 2, pp. 55-177 The Natural History Museum Cromwell Road London SW7 5BD Issued 25 November 1993 Typeset by Ann Buchan (Typesetters), Middlesex Printed in Great Britain at The Alden Press. -
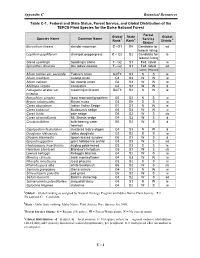
Appendix C Botanical Resources
Appendix C Botanical Resources Table C-1. Federal and State Status, Forest Service, and Global Distribution of the TEPCS Plant Species for the Boise National Forest Forest Global State Global Species Name Common Name 1 2 Service 4 Rank Rank 3 Distrib. Status Botrychium lineare slender moonwort C –G1 SH Candidate for sd federal listing Lepidium papilliferum slickspot peppergrass C – G2 S2 Candidate for le federal listing Silene spaldingii Spalding’s silene T - G2 S1 Fed. listed re Spiranthes diluvialis Ute ladies’-tresses T - G2 S1 Fed. listed sd Current Proposed Allium tolmiei var. persimile Tolmie's onion G4/T3 S3 S S le Allium madidum swamp onion G3 S3 W W re Allium validum tall swamp onion G4 S3 W W re Allotropa virgata candystick G4 S3 W W d Astragalus atratus var. mourning milkvetch G4/T3 S3 N W le inceptus Botrychium simplex least moonwort/grapefern G5 S2 N S w Bryum calobryoides Bryum moss G3 SH S S w Carex aboriginum Indian Valley Sedge G1 S1 N W le Carex bubaumii Buxbaum's sedge G5 S3 W W w Carex livida pale sedge G5 S2 W S cb Carex straminiformis Mt. Shasta sedge G4 S2 W S d Cicuta bulbifera bulb-bearing water G5 S2 W S d hemlock Cypripedium fasiculatum clustered lady’s-slipper G4 S3 N W d Douglasia idahoensis Idaho douglasia G2 S2 S S re Drosera intermedia spoon-leaved sundew G5 S1 W S d Epipactis gigantea giant helleborine orchid G3 S3 W S sd Haplopappus insecticruris bugleg goldenweed G3 S3 S S le Helodium blandowii Blandow's helodium G5 S1 W S cb Lewisia kelloggii Kellogg's bitteroot G4 S2 W S re Mimulus clivicola bank monkeyflower G4 S3 W W re Phacelia minutissma least phacelia G3 S2 S S re Rhynchospora alba white beakbrush G5 S2 W S cb Sanicula graveolens Sierra sanicle G4 S1 N W w Scheuchzeria palustris pod grass G5 S2 W S w Sedum borschii Borch's stonecrop G3 S2 W S sd Sphaeromeria potentilloides cinquefoil tansy G5 S1 N W le Stylocline filaginea stylocline G4 S2 W S re 1Global - Global ranking as assigned by Natural Heritage Program and Idaho Native Plant Society. -

Dedicated to the Preservation of the California Native Flora
DEDICATED TO THE PRESERVATION OF THE CALIFORNIA NATIVE FLORA NEWSLETTER Vol. 11, No. 5 September 19 NEXT MEETING September 30, 7:30 p.m., at the Sierra Baptist Church, 346 North Edwards, Independence. Terry Hicks of the U.S. Forest Service will spea on the plant survey in the White Mountains. PRESIDENT'S MESSAGE: Hats off to the great group of people who were the members and guests of the Bristlecone Chapter on a recent field trip to the White Mountains. Twa cars had problems. Everyone present was more than willing to do whatever was necessary to ensure that all present had a good time. Some trip members had brought tools and a tow rope, others lent a shoulder to push, some made room in their vehicles for unexpected luggage and Dassengers, and others waited patiently for all of this work to be done. This spirit of cooperation more often manifests itself as the experienced members with scientific names on the tips,,oftheir tongues spell them out and patiently wait while they are laborously written down. The professionals among us often share their data in publications as well as in the field. In these times of electioneering backbiting, gang violence, and widespread political unrest, this mutual aid attitude is most refreshing. Keep it up! Thanks to all. Evelyn Mae Nikolaus PROPOSED SLATE OF OFFICERS for 1993 President Betty Gilchrist Vice-president Carla Scheidlinger Secretary Sally Manning Treasurer Scott Hetzler Page 2 FIELD TRIP REPORTS JUNE 27, Little Onion Valley, west of Independence. The day dawned warm and clear as some seventeen multigenerational members and visitors met in Independence for the short drive To The locked gate guarding the Rex Montis Mine road. -

A Molecular Phylogenetic Approach to Western North America Endemic a Rtemisia and Allies (Asteraceae): Untangling the Sagebrushes 1
American Journal of Botany 98(4): 638–653. 2011. A MOLECULAR PHYLOGENETIC APPROACH TO WESTERN NORTH AMERICA ENDEMIC A RTEMISIA AND ALLIES (ASTERACEAE): 1 UNTANGLING THE SAGEBRUSHES 2,6 3 4 3 S ò nia Garcia , E. Durant McArthur , Jaume Pellicer , Stewart C. Sanderson , Joan Vall è s 5 , and Teresa Garnatje 2 2 Institut Bot à nic de Barcelona (IBB-CSIC-ICUB). Passeig del Migdia s/n 08038 Barcelona, Catalonia, Spain; 3 Shrub Sciences Laboratory, Rocky Mountain Research Station, Forest Service, United States Department of Agriculture, Provo, Utah 84606 USA; 4 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, United Kingdom; and 5 Laboratori de Bot à nica, Facultat de Farm à cia, Universitat de Barcelona. Av. Joan XXIII s/n 08028 Barcelona, Catalonia, Spain • Premise of the study : Artemisia subgenus Tridentatae plants characterize the North American Intermountain West. These are landscape-dominant constituents of important ecological communities and habitats for endemic wildlife. Together with allied species and genera ( Picrothamnus and Sphaeromeria ), they make up an intricate series of taxa whose limits are uncertain, likely the result of reticulate evolution. The objectives of this study were to resolve relations among Tridentatae species and their near relatives by delimiting the phylogenetic positions of subgenus Tridentatae species with particular reference to its New World geographic placement and to provide explanations for the relations of allied species and genera with the subgenus with an assessment of their current taxonomic placement. • Methods : Bayesian inference and maximum parsimony analysis were based on 168 newly generated sequences (including the nuclear ITS and ETS and the plastid trnS UGA - trnfM CAU and trnS GCU - trnC GCA ) and 338 previously published sequences (ITS and ETS). -

I INDIVIDUALISTIC and PHYLOGENETIC PERSPECTIVES ON
INDIVIDUALISTIC AND PHYLOGENETIC PERSPECTIVES ON PLANT COMMUNITY PATTERNS Jeffrey E. Ott A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biology Chapel Hill 2010 Approved by: Robert K. Peet Peter S. White Todd J. Vision Aaron Moody Paul S. Manos i ©2010 Jeffrey E. Ott ALL RIGHTS RESERVED ii ABSTRACT Jeffrey E. Ott Individualistic and Phylogenetic Perspectives on Plant Community Patterns (Under the direction of Robert K. Peet) Plant communities have traditionally been viewed as spatially discrete units structured by dominant species, and methods for characterizing community patterns have reflected this perspective. In this dissertation, I adopt an an alternative, individualistic community characterization approach that does not assume discreteness or dominant species importance a priori (Chapter 2). This approach was used to characterize plant community patterns and their relationship with environmental variables at Zion National Park, Utah, providing details and insights that were missed or obscure in previous vegetation characterizations of the area. I also examined community patterns at Zion National Park from a phylogenetic perspective (Chapter 3), under the assumption that species sharing common ancestry should be ecologically similar and hence be co-distributed in predictable ways. I predicted that related species would be aggregated into similar habitats because of phylogenetically-conserved niche affinities, yet segregated into different plots because of competitive interactions. However, I also suspected that these patterns would vary between different lineages and at different levels of the phylogenetic hierarchy (phylogenetic scales). I examined aggregation and segregation in relation to null models for each pair of species within genera and each sister pair of a genus-level vascular plant iii supertree.