Factors Affecting the Abundance of Paracyathus Stearns!! on Subtidal Rock Walls
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Sexual Reproduction of the Solitary Sunset Cup Coral Leptopsammia Pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean
Marine Biology (2005) 147: 485–495 DOI 10.1007/s00227-005-1567-z RESEARCH ARTICLE S. Goffredo Æ J. Radetic´Æ V. Airi Æ F. Zaccanti Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis Received: 16 July 2004 / Accepted: 18 December 2004 / Published online: 3 March 2005 Ó Springer-Verlag 2005 Abstract Information on the reproduction in scleractin- came indented, assuming a sickle or dome shape. We can ian solitary corals and in those living in temperate zones hypothesize that the nucleus’ migration and change of is notably scant. Leptopsammia pruvoti is a solitary coral shape may have to do with facilitating fertilization and living in the Mediterranean Sea and along Atlantic determining the future embryonic axis. During oogene- coasts from Portugal to southern England. This coral sis, oocyte diameter increased from a minimum of 20 lm lives in shaded habitats, from the surface to 70 m in during the immature stage to a maximum of 680 lm depth, reaching population densities of >17,000 indi- when mature. Embryogenesis took place in the coelen- viduals mÀ2. In this paper, we discuss the morphological teron. We did not see any evidence that even hinted at aspects of sexual reproduction in this species. In a sep- the formation of a blastocoel; embryonic development arate paper, we report the quantitative data on the an- proceeded via stereoblastulae with superficial cleavage. nual reproductive cycle and make an interspecific Gastrulation took place by delamination. Early and late comparison of reproductive traits among Dend- embryos had diameters of 204–724 lm and 290–736 lm, rophylliidae aimed at defining different reproductive respectively. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

The Diet and Predator-Prey Relationships of the Sea Star Pycnopodia Helianthoides (Brandt) from a Central California Kelp Forest
THE DIET AND PREDATOR-PREY RELATIONSHIPS OF THE SEA STAR PYCNOPODIA HELIANTHOIDES (BRANDT) FROM A CENTRAL CALIFORNIA KELP FOREST A Thesis Presented to The Faculty of Moss Landing Marine Laboratories San Jose State University In Partial Fulfillment of the Requirements for the Degree Master of Arts by Timothy John Herrlinger December 1983 TABLE OF CONTENTS Acknowledgments iv Abstract vi List of Tables viii List of Figures ix INTRODUCTION 1 MATERIALS AND METHODS Site Description 4 Diet 5 Prey Densities and Defensive Responses 8 Prey-Size Selection 9 Prey Handling Times 9 Prey Adhesion 9 Tethering of Calliostoma ligatum 10 Microhabitat Distribution of Prey 12 OBSERVATIONS AND RESULTS Diet 14 Prey Densities 16 Prey Defensive Responses 17 Prey-Size Selection 18 Prey Handling Times 18 Prey Adhesion 19 Tethering of Calliostoma ligatum 19 Microhabitat Distribution of Prey 20 DISCUSSION Diet 21 Prey Densities 24 Prey Defensive Responses 25 Prey-Size Selection 27 Prey Handling Times 27 Prey Adhesion 28 Tethering of Calliostoma ligatum and Prey Refugia 29 Microhabitat Distribution of Prey 32 Chemoreception vs. a Chemotactile Response 36 Foraging Strategy 38 LITERATURE CITED 41 TABLES 48 FIGURES 56 iii ACKNOWLEDGMENTS My span at Moss Landing Marine Laboratories has been a wonderful experience. So many people have contributed in one way or another to the outcome. My diving buddies perse- vered through a lot and I cherish our camaraderie: Todd Anderson, Joel Thompson, Allan Fukuyama, Val Breda, John Heine, Mike Denega, Bruce Welden, Becky Herrlinger, Al Solonsky, Ellen Faurot, Gilbert Van Dykhuizen, Ralph Larson, Guy Hoelzer, Mickey Singer, and Jerry Kashiwada. Kevin Lohman and Richard Reaves spent many hours repairing com puter programs for me. -

Kelp Forest Monitoring Handbook — Volume 1: Sampling Protocol
KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL CHANNEL ISLANDS NATIONAL PARK KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL Channel Islands National Park Gary E. Davis David J. Kushner Jennifer M. Mondragon Jeff E. Mondragon Derek Lerma Daniel V. Richards National Park Service Channel Islands National Park 1901 Spinnaker Drive Ventura, California 93001 November 1997 TABLE OF CONTENTS INTRODUCTION .....................................................................................................1 MONITORING DESIGN CONSIDERATIONS ......................................................... Species Selection ...........................................................................................2 Site Selection .................................................................................................3 Sampling Technique Selection .......................................................................3 SAMPLING METHOD PROTOCOL......................................................................... General Information .......................................................................................8 1 m Quadrats ..................................................................................................9 5 m Quadrats ..................................................................................................11 Band Transects ...............................................................................................13 Random Point Contacts ..................................................................................15 -

The Earliest Diverging Extant Scleractinian Corals Recovered by Mitochondrial Genomes Isabela G
www.nature.com/scientificreports OPEN The earliest diverging extant scleractinian corals recovered by mitochondrial genomes Isabela G. L. Seiblitz1,2*, Kátia C. C. Capel2, Jarosław Stolarski3, Zheng Bin Randolph Quek4, Danwei Huang4,5 & Marcelo V. Kitahara1,2 Evolutionary reconstructions of scleractinian corals have a discrepant proportion of zooxanthellate reef-building species in relation to their azooxanthellate deep-sea counterparts. In particular, the earliest diverging “Basal” lineage remains poorly studied compared to “Robust” and “Complex” corals. The lack of data from corals other than reef-building species impairs a broader understanding of scleractinian evolution. Here, based on complete mitogenomes, the early onset of azooxanthellate corals is explored focusing on one of the most morphologically distinct families, Micrabaciidae. Sequenced on both Illumina and Sanger platforms, mitogenomes of four micrabaciids range from 19,048 to 19,542 bp and have gene content and order similar to the majority of scleractinians. Phylogenies containing all mitochondrial genes confrm the monophyly of Micrabaciidae as a sister group to the rest of Scleractinia. This topology not only corroborates the hypothesis of a solitary and azooxanthellate ancestor for the order, but also agrees with the unique skeletal microstructure previously found in the family. Moreover, the early-diverging position of micrabaciids followed by gardineriids reinforces the previously observed macromorphological similarities between micrabaciids and Corallimorpharia as -

Using DNA Barcoding Technique to Identify Some Scleractinian Coral Species Along the Egyptian Coast of the Red Sea and Southern of Arabian Gulf
Journal of Environmental Science and Public Health doi: 10.26502/jesph.96120028 Volume 2, Issue 1 Research Article Using DNA Barcoding Technique to Identify Some Scleractinian Coral Species along the Egyptian Coast of the Red Sea and Southern of Arabian Gulf Mohammed Ahmed Sadek1*, Mohammed Ismail Ahmed2, Fedekar Fadel Madkour1 and Mahmoud Hasan Hanafy2 1Department of Marine Science, Faculty of Science, Port Said University, Port Said, Egypt 2Department Marine Science, Faculty of Science, Suez Canal University, Ismailia, Egypt *Corresponding Author: Mohammed Ahmed Sadek, Department of Marine Science, Faculty of Science, Port Said University, Port Said, Egypt, Tel: 00971501092572; E-mail: [email protected] Received: 05 February 2018; Accepted: 22 February 2018; Published: 28 February 2018 Abstract Scleractinian corals Phylogenetic studies have been shown that most taxa are not matched with their evolutionary histories. Using gross morphology as principal base, traditional taxonomy cannot solve the lack of well-defined and homologous characters that can describe scleractinian diversity sufficient. Scleractinian Coral species are hard to identify because of their morphological plasticity. DNA barcoding techniques were used to build molecular phylogenetic analysis to some common scleractinian species in Red Sea and Arabian Gulf. The phylogenetic analysis showed that Porites harrisoni, was clustered separate from other Porites sp collected from gene bank. While for Acropora digitefra genetic divergences were recorded between samples collected from the Red Sea and Gulf of Aqaba. Platygyra daeleda sample from Fanous (Red Sea) were distinct from other Platygyra daeleda samples same case were recorded with Pocillopora verrucosa samples. The DNA barcoding technique proves to be very useful in not only differentiating between species but also finding genetic diversity within species. -
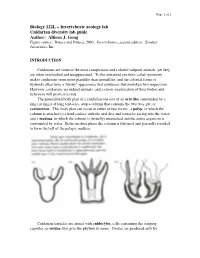
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

Spatial Distribution and the Effects of Competition on Some Temperate Scleractinia and Corallimorpharia
MARINE ECOLOGY PROGRESS SERIES Vol. 70: 39-48, 1991 Published February 14 Mar. Ecol. Prog. Ser. ~ Spatial distribution and the effects of competition on some temperate Scleractinia and Corallimorpharia Nanette E. Chadwick* Department of Zoology. University of California, Berkeley. California 94720. USA ABSTRACT: The impact of interference competition on coral community structure is poorly understood. On subtidal rocks in the northeastern Pacific, members of 3 scleractinian coral species (Astrangia lajollaensis, Balanophyllia elegans, Paracyathus stearnsii) and 1 corallimorpharian (Corynactls califor- nica) were examined to determine whether competition exerts substantial influence over the~rabund- ance and distributional patterns. These anthozoans occupy > 50 % cover on hard substrata, and exhibit characteristic patterns of spatial distribution, with vertical zonation and segregation among some species. They interact in an interspecific dominance hierarchy that lacks reversals, and is linear and consistent under laboratory and field conditions. Experiments demonstrated that a competitive domin- ant, C. californica, influences the abundance and population structure of a subordinate. B. elegans, by (1)reducing sexual reproductive output, (2) increasing larval mortality, (3)altering recruitment patterns. Field cross-transplants revealed that the dominant also affects vertical zonation of a competitive intermediate, A. lajollaensis, by lulling polyps that occur near the tops of subtidal rocks. It is concluded that between-species competition, -

Equatorial Submergence in a Solitary Coral, Balanophyllia Elegans, and the Critical Life Stage Excluding the Species from Shallow Water in the South
MARINE ECOLOGY - PROGRESS SERIES Vol. 1.227-235, 1979 hblished November 30 1 Mar. Ecol. Prog. Ser. Equatorial Submergence in a Solitary Coral, Balanophyllia elegans, and the Critical Life Stage Excluding the Species from Shallow Water in the South T. Gerrodette Scripps Institution of Oceanography. University of California, La Jolla, California 92093, USA ABSTRACT: The geographic and bathymetric distributions of a solitary coral, Balanophyllia elegans, were studied, especially in the southern part of its range. This species is an example of equatorial submergence, occurring intertidally and subtidally in the north (Vancouver Island, Canada, to Point Conception, California), but subtidally only In the south (Point Conception to northern Baja Cal~fornla,Mexico). The absence of B elegans from depths less than 10 m in the south was investigated to identify the life history stages most sensitive to environmental factors, particularly temperature, in shallow water Newly settled and adult individuals were transplanted to buoys in the top few meters of water The transplanted adults grew more slowly than controls, but survived well and reproduced; moreover, planula larvae released by the transplanted adults successfully settled nearby under shallow-water conditions. Thus neither very early nor relatively late stages In the life history appeared cr~ticalin excluding B. elegans from shallow water. The critical period was the juvenile stage, since newly settled individuals showed poor survival after transplantation to buoys in shallow water. Distribution and mortality patterns were correlated with the temperature of the seawater. However, it is difficult to establish a simple causal relationship between high temperature and equatorial submergence, since subtidal temperature patterns are complex and since temperature may have many indirect but important effects. -

Spectral Diversity of Fluorescent Proteins from the Anthozoan Corynactis Californica
Mar Biotechnol (2008) 10:328–342 DOI 10.1007/s10126-007-9072-7 ORIGINAL ARTICLE Spectral Diversity of Fluorescent Proteins from the Anthozoan Corynactis californica Christine E. Schnitzler & Robert J. Keenan & Robert McCord & Artur Matysik & Lynne M. Christianson & Steven H. D. Haddock Received: 7 September 2007 /Accepted: 19 November 2007 /Published online: 11 March 2008 # Springer Science + Business Media, LLC 2007 Abstract Color morphs of the temperate, nonsymbiotic three to four distinct genetic loci that code for these colors, corallimorpharian Corynactis californica show variation in and one morph contains at least five loci. These genes pigment pattern and coloring. We collected seven distinct encode a subfamily of new GFP-like proteins, which color morphs of C. californica from subtidal locations in fluoresce across the visible spectrum from green to red, Monterey Bay, California, and found that tissue– and color– while sharing between 75% to 89% pairwise amino-acid morph-specific expression of at least six different genes is identity. Biophysical characterization reveals interesting responsible for this variation. Each morph contains at least spectral properties, including a bright yellow protein, an orange protein, and a red protein exhibiting a “fluorescent timer” phenotype. Phylogenetic analysis indicates that the Christine E. Schnitzler and Robert J. Keenan contributed equally to FP genes from this species evolved together but that this work. diversification of anthozoan fluorescent proteins has taken Data deposition footnote: -

1 Population Characteristics of Balanophyllia Elegans in the San
Population Characteristics of Balanophyllia elegans in the San Juan Archipelago Hana Dubail University of Washington ABSTRACT: Determining predictors of Balanophyllia elegans’ population distribution and physical size is important for ecological research in the Eastern Pacific because changes in this species’ biomass, abundance, and environment could be an indication of realized impact from climate change and ocean acidification. Over the last four decades community composition has changed around the San Juan Islands, including a decline in B. elegans’ density. In this study we assessed abundance, biomass, population density, largest diameter, and surface area at 11 sites from the years 2008-2013, at depths ranging from 3 m to 27.5 m with 3 m increments, and at four flow ranks, looking for patterns and predictors. We found that all three variables (site, year, and depth) and the interaction variables ‘site + depth’ and ‘site + year + depth’ are statistically significant predictors for coral distribution, and year, depth, and the interaction term ‘year + site’ are significant predictors for oral disc surface area. These results tell us that these sites are unique, but each site has an interesting pattern that will need to be defined through further analysis for a larger sample size. INTRODUCTION Climate change is causing a global ocean trend towards warmer surface temperatures and decreased pH (Orr et al. 2005). It is important to determine how this is changing ocean ecology for conservation efforts and future predictions of ocean processes, because the ocean’s ability to provide services and commodities to a growing human population is decreasing. Consequences of the increasing rate of greenhouse gas released into the atmosphere are still largely unknown.