Zoologische Mededelingen 78-02
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patterns of Septal Biomineralization in Scleractinia Compared with Their 28S Rrna Phylogeny
PBlackwell Publishingatterns Ltd. of septal biomineralization in Scleractinia compared with their 28S rRNA phylogeny: a dual approach for a new taxonomic framework JEAN-PIERRE CUIF, GUILLAUME LECOINTRE, CHRISTINE PERRIN, ANNIE TILLIER & SIMON TILLIER Accepted: 2 December 2002 Cuif, J.-P., Lecointre, G., Perrin, C., Tillier, A. & Tillier, S. (2003). Patterns of septal bio- mineralization in Scleractinia compared with their 28S rRNA phylogeny: a dual approach for a new taxonomic framework. — Zoologica Scripta, 32, 459–473. A molecular phylogeny of the Scleractinia is reconstructed from approximately 700 nucleo- tides of the 5′end of the 28S rDNA obtained from 40 species. A comparison of molecular phylogenic trees with biomineralization patterns of coral septa suggests that at least five clades are corroborated by both types of data. Agaricidae and Dendrophylliidae are found to be monophyletic, that is supported by microstructural data. Conversely, Faviidae and Caryophyl- liidae are found to be paraphyletic: Cladocora should be excluded from the faviids, whereas Eusmilia should be excluded from the caryophylliids. The conclusion is also supported by the positions, sizes and shapes of centres of calcification. The traditional Guyniidae are diphyletic, corroborating Stolarski’s hypothesis ‘A’. Some results from our most parsimonious trees are not strongly statistically supported but corroborated by other molecular studies and micro- structural observations. For example, in the scleractinian phylogenetic tree, there are several lines of evidence (including those from our data) to distinguish a Faviidae–Mussidae lineage and a Dendrophylliidae–Agaricidae–Poritidae–Siderastreidae lineage. From a methodological standpoint, our results suggest that co-ordinated studies creating links between biomineralization patterns and molecular phylogeny may provide an efficient working approach for a re- examination of scleractinian classification. -

Factors Affecting the Abundance of Paracyathus Stearns!! on Subtidal Rock Walls
MLML / M8~RllIBR;jRY 8272 MOSS LANDING RD. MOSS LANDING, CA 95039 FACTORS AFFECTING THE ABUNDANCE OF PARACYATHUS STEARNS!! ON SUBTIDAL ROCK WALLS by Mark Pranger A thesis submitted in partial fulfillment ofthe requirements for the degree of Master ofScience in Marine Science in the School ofNatural Sciences California State University, Fresno August 1999 ACKNOWLEDGMENTS I would like to thank the members of my graduate advisory committee for their advice and guidance during this process. Dr. James Nybakken for his interest and education in invertebrate biology and for allowing me to help in the teaching of others. For the insights and humor of Dr. Mike Foster, that helped improve this study and my education. For the help of Dr. Stephen Ervin who in the last hours help me through all CSU Fresno's paper work and deadlines. I am grateful to the staff at the Pollution Studies Lab at Granite Canyon for their help during experiments and for allowing me the use of their working space. I am indebted to the staff at Moss Landing Marine Labs that kept all the equipment working and operational, and to the Dr. Earl and Ethel Myers Oceanographic and Marine Biology Trust for helping to fund this project. Most of all, thanks goes to the many students at Moss Landing. Without their help this study would not have been completed. Special thanks goes to; Mat Edwards for being a faithful dive buddy, to Torno Eguchi, Michelle White, Bryn Phillips, Cassandra Roberts, Michelle Lander, and Lara Lovera for their help on statistics and editing of early drafts, and especially to Michele Jacobi for her help in all aspects of this study and her constant encouragement for me to finish. -

The Diet and Predator-Prey Relationships of the Sea Star Pycnopodia Helianthoides (Brandt) from a Central California Kelp Forest
THE DIET AND PREDATOR-PREY RELATIONSHIPS OF THE SEA STAR PYCNOPODIA HELIANTHOIDES (BRANDT) FROM A CENTRAL CALIFORNIA KELP FOREST A Thesis Presented to The Faculty of Moss Landing Marine Laboratories San Jose State University In Partial Fulfillment of the Requirements for the Degree Master of Arts by Timothy John Herrlinger December 1983 TABLE OF CONTENTS Acknowledgments iv Abstract vi List of Tables viii List of Figures ix INTRODUCTION 1 MATERIALS AND METHODS Site Description 4 Diet 5 Prey Densities and Defensive Responses 8 Prey-Size Selection 9 Prey Handling Times 9 Prey Adhesion 9 Tethering of Calliostoma ligatum 10 Microhabitat Distribution of Prey 12 OBSERVATIONS AND RESULTS Diet 14 Prey Densities 16 Prey Defensive Responses 17 Prey-Size Selection 18 Prey Handling Times 18 Prey Adhesion 19 Tethering of Calliostoma ligatum 19 Microhabitat Distribution of Prey 20 DISCUSSION Diet 21 Prey Densities 24 Prey Defensive Responses 25 Prey-Size Selection 27 Prey Handling Times 27 Prey Adhesion 28 Tethering of Calliostoma ligatum and Prey Refugia 29 Microhabitat Distribution of Prey 32 Chemoreception vs. a Chemotactile Response 36 Foraging Strategy 38 LITERATURE CITED 41 TABLES 48 FIGURES 56 iii ACKNOWLEDGMENTS My span at Moss Landing Marine Laboratories has been a wonderful experience. So many people have contributed in one way or another to the outcome. My diving buddies perse- vered through a lot and I cherish our camaraderie: Todd Anderson, Joel Thompson, Allan Fukuyama, Val Breda, John Heine, Mike Denega, Bruce Welden, Becky Herrlinger, Al Solonsky, Ellen Faurot, Gilbert Van Dykhuizen, Ralph Larson, Guy Hoelzer, Mickey Singer, and Jerry Kashiwada. Kevin Lohman and Richard Reaves spent many hours repairing com puter programs for me. -

Kelp Forest Monitoring Handbook — Volume 1: Sampling Protocol
KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL CHANNEL ISLANDS NATIONAL PARK KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL Channel Islands National Park Gary E. Davis David J. Kushner Jennifer M. Mondragon Jeff E. Mondragon Derek Lerma Daniel V. Richards National Park Service Channel Islands National Park 1901 Spinnaker Drive Ventura, California 93001 November 1997 TABLE OF CONTENTS INTRODUCTION .....................................................................................................1 MONITORING DESIGN CONSIDERATIONS ......................................................... Species Selection ...........................................................................................2 Site Selection .................................................................................................3 Sampling Technique Selection .......................................................................3 SAMPLING METHOD PROTOCOL......................................................................... General Information .......................................................................................8 1 m Quadrats ..................................................................................................9 5 m Quadrats ..................................................................................................11 Band Transects ...............................................................................................13 Random Point Contacts ..................................................................................15 -
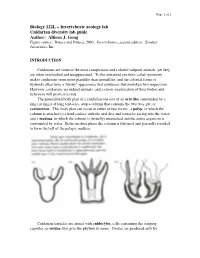
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

CNIDARIA Corals, Medusae, Hydroids, Myxozoans
FOUR Phylum CNIDARIA corals, medusae, hydroids, myxozoans STEPHEN D. CAIRNS, LISA-ANN GERSHWIN, FRED J. BROOK, PHILIP PUGH, ELLIOT W. Dawson, OscaR OcaÑA V., WILLEM VERvooRT, GARY WILLIAMS, JEANETTE E. Watson, DENNIS M. OPREsko, PETER SCHUCHERT, P. MICHAEL HINE, DENNIS P. GORDON, HAMISH J. CAMPBELL, ANTHONY J. WRIGHT, JUAN A. SÁNCHEZ, DAPHNE G. FAUTIN his ancient phylum of mostly marine organisms is best known for its contribution to geomorphological features, forming thousands of square Tkilometres of coral reefs in warm tropical waters. Their fossil remains contribute to some limestones. Cnidarians are also significant components of the plankton, where large medusae – popularly called jellyfish – and colonial forms like Portuguese man-of-war and stringy siphonophores prey on other organisms including small fish. Some of these species are justly feared by humans for their stings, which in some cases can be fatal. Certainly, most New Zealanders will have encountered cnidarians when rambling along beaches and fossicking in rock pools where sea anemones and diminutive bushy hydroids abound. In New Zealand’s fiords and in deeper water on seamounts, black corals and branching gorgonians can form veritable trees five metres high or more. In contrast, inland inhabitants of continental landmasses who have never, or rarely, seen an ocean or visited a seashore can hardly be impressed with the Cnidaria as a phylum – freshwater cnidarians are relatively few, restricted to tiny hydras, the branching hydroid Cordylophora, and rare medusae. Worldwide, there are about 10,000 described species, with perhaps half as many again undescribed. All cnidarians have nettle cells known as nematocysts (or cnidae – from the Greek, knide, a nettle), extraordinarily complex structures that are effectively invaginated coiled tubes within a cell. -

Spatial Distribution and the Effects of Competition on Some Temperate Scleractinia and Corallimorpharia
MARINE ECOLOGY PROGRESS SERIES Vol. 70: 39-48, 1991 Published February 14 Mar. Ecol. Prog. Ser. ~ Spatial distribution and the effects of competition on some temperate Scleractinia and Corallimorpharia Nanette E. Chadwick* Department of Zoology. University of California, Berkeley. California 94720. USA ABSTRACT: The impact of interference competition on coral community structure is poorly understood. On subtidal rocks in the northeastern Pacific, members of 3 scleractinian coral species (Astrangia lajollaensis, Balanophyllia elegans, Paracyathus stearnsii) and 1 corallimorpharian (Corynactls califor- nica) were examined to determine whether competition exerts substantial influence over the~rabund- ance and distributional patterns. These anthozoans occupy > 50 % cover on hard substrata, and exhibit characteristic patterns of spatial distribution, with vertical zonation and segregation among some species. They interact in an interspecific dominance hierarchy that lacks reversals, and is linear and consistent under laboratory and field conditions. Experiments demonstrated that a competitive domin- ant, C. californica, influences the abundance and population structure of a subordinate. B. elegans, by (1)reducing sexual reproductive output, (2) increasing larval mortality, (3)altering recruitment patterns. Field cross-transplants revealed that the dominant also affects vertical zonation of a competitive intermediate, A. lajollaensis, by lulling polyps that occur near the tops of subtidal rocks. It is concluded that between-species competition, -

Bibliography on the Scyphozoa with Selected References on Hydrozoa and Anthozoa
W&M ScholarWorks Reports 1971 Bibliography on the Scyphozoa with selected references on Hydrozoa and Anthozoa Dale R. Calder Virginia Institute of Marine Science Harold N. Cones Virginia Institute of Marine Science Edwin B. Joseph Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons, and the Zoology Commons Recommended Citation Calder, D. R., Cones, H. N., & Joseph, E. B. (1971) Bibliography on the Scyphozoa with selected references on Hydrozoa and Anthozoa. Special scientific eporr t (Virginia Institute of Marine Science) ; no. 59.. Virginia Institute of Marine Science, William & Mary. https://doi.org/10.21220/V59B3R This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BIBLIOGRAPHY on the SCYPHOZOA WITH SELECTED REFERENCES ON HYDROZOA and ANTHOZOA Dale R. Calder, Harold N. Cones, Edwin B. Joseph SPECIAL SCIENTIFIC REPORT NO. 59 VIRGINIA INSTITUTE. OF MARINE SCIENCE GLOUCESTER POINT, VIRGINIA 23012 AUGUST, 1971 BIBLIOGRAPHY ON THE SCYPHOZOA, WITH SELECTED REFERENCES ON HYDROZOA AND ANTHOZOA Dale R. Calder, Harold N. Cones, ar,d Edwin B. Joseph SPECIAL SCIENTIFIC REPORT NO. 59 VIRGINIA INSTITUTE OF MARINE SCIENCE Gloucester Point, Virginia 23062 w. J. Hargis, Jr. April 1971 Director i INTRODUCTION Our goal in assembling this bibliography has been to bring together literature references on all aspects of scyphozoan research. Compilation was begun in 1967 as a card file of references to publications on the Scyphozoa; selected references to hydrozoan and anthozoan studies that were considered relevant to the study of scyphozoans were included. -

Adorable Anemone
inspirationalabout this guide | about anemones | colour index | species index | species pages | icons | glossary invertebratesadorable anemonesa guide to the shallow water anemones of New Zealand Version 1, 2019 Sadie Mills Serena Cox with Michelle Kelly & Blayne Herr 1 about this guide | about anemones | colour index | species index | species pages | icons | glossary about this guide Anemones are found everywhere in the sea, from under rocks in the intertidal zone, to the deepest trenches of our oceans. They are a colourful and diverse group, and we hope you enjoy using this guide to explore them further and identify them in the field. ADORABLE ANEMONES is a fully illustrated working e-guide to the most commonly encountered shallow water species of Actiniaria, Corallimorpharia, Ceriantharia and Zoantharia, the anemones of New Zealand. It is designed for New Zealanders like you who live near the sea, dive and snorkel, explore our coasts, make a living from it, and for those who educate and are charged with kaitiakitanga, conservation and management of our marine realm. It is one in a series of e-guides on New Zealand Marine invertebrates and algae that NIWA’s Coasts and Oceans group is presently developing. The e-guide starts with a simple introduction to living anemones, followed by a simple colour index, species index, detailed individual species pages, and finally, icon explanations and a glossary of terms. As new species are discovered and described, new species pages will be added and an updated version of this e-guide will be made available. Each anemone species page illustrates and describes features that will enable you to differentiate the species from each other. -

The Juwel Anemone Corynactis Viridis , a New Order for the Netherlands
the juwel anemone CORYNACTIS VIRIDIS, a new order for the netherlands (cnidaria: corallimorpharia) Adriaan Gittenberger, Niels Schrieken, Joop Coolen & Wijnand Vlierhuis During an expedition with scuba-divers to the Dutch part of the Brown Ridge in the central North Sea in June 2013, two colonies of the jewel anemone Corynactis viridis were found on the wreck Anna Graebe. With the jewel anemone both a new species and a new animal order, the Corallimorpharia, are added to the autochthonous fauna of the Netherlands. This species typically occurs in the Mediterranean and along the Atlantic coast from Portugal and the west British Isles up to Shetland. As other records of settled colonies of C. viridis in the North Sea were recently reported from Belgian, German and English waters, it is concluded that the jewel anemone, which used to be known as an occasional visitor, should now be considered autochthonous in the North Sea. the first record of live C. viridis in the Nether- introduction lands, it was not proof of its autochthonous On August 6th 1960 the jewel anemone Corynactis occurrence. Thongweed is a common species in viridis Allman, 1846 was recorded as new to the drift assemblages, but is not known to be settled Netherlands by den Hartog (1960). Four polyps in Dutch waters. The individuals that commonly were found attached to the roots of a thongweed, wash ashore therefore must come from abroad, Himanthalia elongata (Linnaeus) S.F. Gray, most likely from the French or English coasts, washed ashore off Den Helder. Although this is drifting along with the south to north current. -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Anemones, including Corynactis viridis, crustose sponges and colonial ascidians on very exposed or wave surged vertical infralittoral rock MarLIN – Marine Life Information Network Marine Evidence–based Sensitivity Assessment (MarESA) Review John Readman 2016-07-03 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/habitats/detail/1120]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Readman, J.A.J., 2016. Anemones, including [Corynactis viridis,] crustose sponges and colonial ascidians on very exposed or wave surged vertical infralittoral rock. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinhab.1120.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions -

(Adriatic Sea, Croatia). 1
NAT. CROAT. VOL. 11 No 3 265¿292 ZAGREB September 30, 2002 ISSN 1330-0520 original scientific paper / izvorni znanstveni rad UDK 591.9:593.6(497.5/(262.3)(1–13) MARINE FAUNA OF THE MLJET NATIONAL PARK (ADRIATIC SEA, CROATIA). 1. ANTHOZOA PETAR KRU@I] Faculty of Science, Department of Zoology, Rooseveltov trg 6, 10000 Zagreb, Croatia ([email protected]) Thais – Society for Exploration and Conservation of Nature, Primorska 23, 10000 Zagreb, Croatia Kru`i}, P.: Marine fauna of the Mljet National Park (Adriatic Sea, Croatia). 1. Anthozoa. Nat. Croat., Vol. 11, No. 3., 265–292, 2002, Zagreb. Fifty-two anthozoan species were recorded and collected in the area of Mljet National Park dur- ing surveys from 1995 to 1998. General and ecological data are presented for each species, as well as distribution and local abundance. Recorded species account for about 60% of anthozoans known in the Adriatic Sea, and for about 45% of anthozoans known in the Mediterranean Sea. Eight of these species were not recorded previously in the Adriatic Sea. Eleven species are considered to be Mediterranean endemics. The heterogeneity of substrates and benthic communities is considerable in the Mljet National Park, with anthozoans present on most different kinds of substrates and in a wide range of benthic communities. Remarkably, the colonial coral Cladocora caespitosa builds a large »reef-like« structure in the Veliko Jezero, in the area characterized by strong bottom hydro- dynamism. Key words: marine fauna, Anthozoa, Mljet, Adriatic Sea Kru`i}, P.: Morska fauna Nacionalnog parka Mljet (Jadransko more, Hrvatska). 1.