The National Bureau of Standards, 1950-1969
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ira Sprague Bowen Papers, 1940-1973
http://oac.cdlib.org/findaid/ark:/13030/tf2p300278 No online items Inventory of the Ira Sprague Bowen Papers, 1940-1973 Processed by Ronald S. Brashear; machine-readable finding aid created by Gabriela A. Montoya Manuscripts Department The Huntington Library 1151 Oxford Road San Marino, California 91108 Phone: (626) 405-2203 Fax: (626) 449-5720 Email: [email protected] URL: http://www.huntington.org/huntingtonlibrary.aspx?id=554 © 1998 The Huntington Library. All rights reserved. Observatories of the Carnegie Institution of Washington Collection Inventory of the Ira Sprague 1 Bowen Papers, 1940-1973 Observatories of the Carnegie Institution of Washington Collection Inventory of the Ira Sprague Bowen Paper, 1940-1973 The Huntington Library San Marino, California Contact Information Manuscripts Department The Huntington Library 1151 Oxford Road San Marino, California 91108 Phone: (626) 405-2203 Fax: (626) 449-5720 Email: [email protected] URL: http://www.huntington.org/huntingtonlibrary.aspx?id=554 Processed by: Ronald S. Brashear Encoded by: Gabriela A. Montoya © 1998 The Huntington Library. All rights reserved. Descriptive Summary Title: Ira Sprague Bowen Papers, Date (inclusive): 1940-1973 Creator: Bowen, Ira Sprague Extent: Approximately 29,000 pieces in 88 boxes Repository: The Huntington Library San Marino, California 91108 Language: English. Provenance Placed on permanent deposit in the Huntington Library by the Observatories of the Carnegie Institution of Washington Collection. This was done in 1989 as part of a letter of agreement (dated November 5, 1987) between the Huntington and the Carnegie Observatories. The papers have yet to be officially accessioned. Cataloging of the papers was completed in 1989 prior to their transfer to the Huntington. -

Revisiting One World Or None
Revisiting One World or None. Sixty years ago, atomic energy was new and the world was still reverberating from the shocks of the atomic bombings of Hiroshima and Nagasaki. Immediately after the end of the Second World War, the first instinct of the administration and Congress was to protect the “secret” of the atomic bomb. The atomic scientists—what today would be called nuclear physicists— who had worked on the Manhattan project to develop atomic bombs recognized that there was no secret, that any technically advanced nation could, with adequate resources, reproduce what they had done. They further believed that when a single aircraft, eventually a single missile, armed with a single bomb could destroy an entire city, no defense system would ever be adequate. Given this combination, the world seemed headed for a period of unprecedented danger. Many of the scientists who had worked on the Manhattan Project felt a special responsibility to encourage a public debate about these new and terrifying products of modern science. They formed an organization, the Federation of Atomic Scientists, dedicated to avoiding the threat of nuclear weapons and nuclear proliferation. Convinced that neither guarding the nuclear “secret” nor any defense could keep the world safe, they encouraged international openness in nuclear physics research and in the expected nuclear power industry as the only way to avoid a disastrous arms race in nuclear weapons. William Higinbotham, the first Chairman of the Association of Los Alamos Scientists and the first Chairman of the Federation of Atomic Scientists, wanted to expand the organization beyond those who had worked on the Manhattan Project, for two reasons. -

Copyright by Paul Harold Rubinson 2008
Copyright by Paul Harold Rubinson 2008 The Dissertation Committee for Paul Harold Rubinson certifies that this is the approved version of the following dissertation: Containing Science: The U.S. National Security State and Scientists’ Challenge to Nuclear Weapons during the Cold War Committee: —————————————————— Mark A. Lawrence, Supervisor —————————————————— Francis J. Gavin —————————————————— Bruce J. Hunt —————————————————— David M. Oshinsky —————————————————— Michael B. Stoff Containing Science: The U.S. National Security State and Scientists’ Challenge to Nuclear Weapons during the Cold War by Paul Harold Rubinson, B.A.; M.A. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin August 2008 Acknowledgements Thanks first and foremost to Mark Lawrence for his guidance, support, and enthusiasm throughout this project. It would be impossible to overstate how essential his insight and mentoring have been to this dissertation and my career in general. Just as important has been his camaraderie, which made the researching and writing of this dissertation infinitely more rewarding. Thanks as well to Bruce Hunt for his support. Especially helpful was his incisive feedback, which both encouraged me to think through my ideas more thoroughly, and reined me in when my writing overshot my argument. I offer my sincerest gratitude to the Smith Richardson Foundation and Yale University International Security Studies for the Predoctoral Fellowship that allowed me to do the bulk of the writing of this dissertation. Thanks also to the Brady-Johnson Program in Grand Strategy at Yale University, and John Gaddis and the incomparable Ann Carter-Drier at ISS. -
![I. I. Rabi Papers [Finding Aid]. Library of Congress. [PDF Rendered Tue Apr](https://docslib.b-cdn.net/cover/8589/i-i-rabi-papers-finding-aid-library-of-congress-pdf-rendered-tue-apr-428589.webp)
I. I. Rabi Papers [Finding Aid]. Library of Congress. [PDF Rendered Tue Apr
I. I. Rabi Papers A Finding Aid to the Collection in the Library of Congress Manuscript Division, Library of Congress Washington, D.C. 1992 Revised 2010 March Contact information: http://hdl.loc.gov/loc.mss/mss.contact Additional search options available at: http://hdl.loc.gov/loc.mss/eadmss.ms998009 LC Online Catalog record: http://lccn.loc.gov/mm89076467 Prepared by Joseph Sullivan with the assistance of Kathleen A. Kelly and John R. Monagle Collection Summary Title: I. I. Rabi Papers Span Dates: 1899-1989 Bulk Dates: (bulk 1945-1968) ID No.: MSS76467 Creator: Rabi, I. I. (Isador Isaac), 1898- Extent: 41,500 items ; 105 cartons plus 1 oversize plus 4 classified ; 42 linear feet Language: Collection material in English Location: Manuscript Division, Library of Congress, Washington, D.C. Summary: Physicist and educator. The collection documents Rabi's research in physics, particularly in the fields of radar and nuclear energy, leading to the development of lasers, atomic clocks, and magnetic resonance imaging (MRI) and to his 1944 Nobel Prize in physics; his work as a consultant to the atomic bomb project at Los Alamos Scientific Laboratory and as an advisor on science policy to the United States government, the United Nations, and the North Atlantic Treaty Organization during and after World War II; and his studies, research, and professorships in physics chiefly at Columbia University and also at Massachusetts Institute of Technology. Selected Search Terms The following terms have been used to index the description of this collection in the Library's online catalog. They are grouped by name of person or organization, by subject or location, and by occupation and listed alphabetically therein. -

UC San Diego UC San Diego Electronic Theses and Dissertations
UC San Diego UC San Diego Electronic Theses and Dissertations Title The new prophet : Harold C. Urey, scientist, atheist, and defender of religion Permalink https://escholarship.org/uc/item/3j80v92j Author Shindell, Matthew Benjamin Publication Date 2011 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, SAN DIEGO The New Prophet: Harold C. Urey, Scientist, Atheist, and Defender of Religion A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in History (Science Studies) by Matthew Benjamin Shindell Committee in charge: Professor Naomi Oreskes, Chair Professor Robert Edelman Professor Martha Lampland Professor Charles Thorpe Professor Robert Westman 2011 Copyright Matthew Benjamin Shindell, 2011 All rights reserved. The Dissertation of Matthew Benjamin Shindell is approved, and it is acceptable in quality and form for publication on microfilm and electronically: ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ Chair University of California, San Diego 2011 iii TABLE OF CONTENTS Signature Page……………………………………………………………………...... iii Table of Contents……………………………………………………………………. iv Acknowledgements…………………………………………………………………. -

The Endless Frontier 75Th Anniversary Edition
the endless frontier 75th Anniversary Edition VANNEVAR BUSH Reprinted in celebration of the National Science Foundation’s 70th anniversary1 | 1950-2020 Book cover photo: © Arnold Newman Collection via Getty Images SCIENCE THE ENDLESS FRONTIER A Report to the President by VANNEVAR BUSH Director of the Ofce of Scientifc Research and Development July 1945 Foreword by France A. Crdova, 14th Director of NSF Reissued by the National Science Foundation in celebration of the agency’s 70th anniversary and the 75th anniversary of Science—the Endless Frontier CELEBRATING NSF’S 70th BIRTHDAY By France A. Crdova, 14th Director of NSF “...basic research is the pacemaker of technological progress.” That statement is as relevant today as it was in 1945 when Vannevar Bush wrote it in his landmark tract, Science—the Endless Frontier. In the report, submitted to President Harry S. Truman, Bush made the case for creating a new agency that he and others felt was needed to support the underlying basic research essential for combatting disease, ensuring national security, and increasing the standard of living, including supporting new industries and jobs. Bush drew an important lesson from directing the Office of Scientific Research and Development during World War II: “The most important ways in which the Government can promote industrial research are to increase the flow of new scientific knowledge through support of basic research and to aid in the development of scientific talent.” Bush desired that the benefits of scientific re- search realized during the war could have even wider application postwar. He advocated for government funding of basic research in universities, colleges, and research institutes because that’s where the talent was. -

Book Review Tuxedo Park, by Jennet Conant (Simon & Schuster, 2002), 330 Pp., ISBN 0684872870, $26.00
Book Review Tuxedo Park, by Jennet Conant (Simon & Schuster, 2002), 330 pp., ISBN 0684872870, $26.00 Reviewed by Jane A. Roman Department of Chemistry, Regis College, Weston, MA In the early chapters of this book, Jennet Conant, granddaughter of James Conant former President of Harvard and esteemed scientist, describes brilliantly the life of Alfred Lee Loomis, philanthropist, scientist and Wall Street tycoon. Prompted by the mysterious circumstances surrounding the suicide of her granduncle, William Richards, son of Nobel laureate Theodore William Richards, Jennet using her granduncles’ notes and letters, wrote this biography, which is a small but significant chapter in the history of American science. Her accounts of Loomis depict his relationships with many Nobel Laureates in science and also detail an illicit love affair Loomis had with Manette Hobart, the wife of Garrett A. Hobart III, his protégé and secretary at Tuxedo Park. The setting for most of the book is the region of Tuxedo Park in Orange County, New York where Loomis established a research laboratory, funded solely by his personal fortune. Very important discoveries in radar detection, atomic fission, and other wartime inventions that led the allies to victory over the Germans were made there. Because the circumstances surrounding her granduncle’s death and the hush-up carried out by her family were indicative of the gentry at that time, Ms. Conant was prompted to reveal in her novel the relationship her granduncle had with Loomis in Tuxedo Park. From his papers and letters, she describes how Richards and his friend at Princeton, George Kistiakowsky, came to work at Tuxedo Park, often referring to it as a private scientific playground in the Ramapo Mountains. -
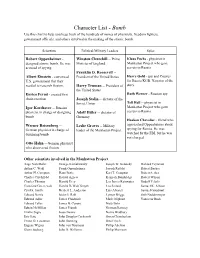
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

ABSTRACT Title of Dissertation: the PRINCIPAL UNCERTAINTY: U.S
ABSTRACT Title of Dissertation: THE PRINCIPAL UNCERTAINTY: U.S. ATOMIC INTELLIGENCE, 1942-1949 Vincent Jonathan Houghton, Doctor of Philosophy, 2013 Dissertation directed by: Professor Jon T. Sumida Department of History The subject of this dissertation is the U. S. atomic intelligence effort against both Nazi Germany and the Soviet Union in the period 1942-1949. Both of these intelligence efforts operated within the framework of an entirely new field of intelligence: scientific intelligence. Because of the atomic bomb, for the first time in history a nation’s scientific resources – the abilities of its scientists, the state of its research institutions and laboratories, its scientific educational system – became a key consideration in assessing a potential national security threat. Considering how successfully the United States conducted the atomic intelligence effort against the Germans in the Second World War, why was the United States Government unable to create an effective atomic intelligence apparatus to monitor Soviet scientific and nuclear capabilities? Put another way, why did the effort against the Soviet Union fail so badly, so completely, in all potential metrics – collection, analysis, and dissemination? In addition, did the general assessment of German and Soviet science lead to particular assumptions about their abilities to produce nuclear weapons? How did this assessment affect American presuppositions regarding the German and Soviet strategic threats? Despite extensive historical work on atomic intelligence, the current historiography has not adequately addressed these questions. THE PRINCIPAL UNCERTAINTY: U.S. ATOMIC INTELLIGENCE, 1942-1949 By Vincent Jonathan Houghton Dissertation submitted to the Faculty of the Graduate School of the University of Maryland, College Park, in partial fulfillment of the requirements for the degree of Doctor of Philosophy 2013 Advisory Committee: Professor Jon T. -

Newly Opened Correspondence Illuminates Einstein's Personal Life
CENTER FOR HISTORY OF PHYSICS NEWSLETTER Vol. XXXVIII, Number 2 Fall 2006 One Physics Ellipse, College Park, MD 20740-3843, Tel. 301-209-3165 Newly Opened Correspondence Illuminates Einstein’s Personal Life By David C. Cassidy, Hofstra University, with special thanks to Diana Kormos Buchwald, Einstein Papers Project he Albert Einstein Archives at the Hebrew University of T Jerusalem recently opened a large collection of Einstein’s personal correspondence from the period 1912 until his death in 1955. The collection consists of nearly 1,400 items. Among them are about 300 letters and cards written by Einstein, pri- marily to his second wife Elsa Einstein, and some 130 letters Einstein received from his closest family members. The col- lection had been in the possession of Einstein’s step-daughter, Margot Einstein, who deposited it with the Hebrew University of Jerusalem with the stipulation that it remain closed for twen- ty years following her death, which occurred on July 8, 1986. The Archives released the materials to public viewing on July 10, 2006. On the same day Princeton University Press released volume 10 of The Collected Papers of Albert Einstein, con- taining 148 items from the collection through December 1920, along with other newly available correspondence. Later items will appear in future volumes. “These letters”, write the Ein- stein editors, “provide the reader with substantial new source material for the study of Einstein’s personal life and the rela- tionships with his closest family members and friends.” H. Richard Gustafson playing with a guitar to pass the time while monitoring the control room at a Fermilab experiment. -

Crossing the Rubicon Barton 1
Crossing the Rubicon Barton 1. Bernstein A Missed Opportunity to Stop the H-Bomb? are in some way limited, the future of our society will come increasingly into peril of the gravest kind. --Panel of Consultants on Disarmament, September 1952 Downloaded from http://direct.mit.edu/isec/article-pdf/14/2/132/694430/isec.14.2.132.pdf by guest on 24 September 2021 That first thermonuclear] test ended the possibility of the only type of agreement that 1 thought was possible with Russia . an agreement to make no more tests. [It]would have been self-policing. 1 still think that we made a grave error in conducting that test at that time. Those who pushed that thing . without making that attempt have a great deal to answer for.’ -Vannevar Bush, April 1954 “In the thermonuclear tests at Eniwetok,” President Harry S. Truman announced in his January 1953 State of the Union address, “we have entered another stage in the worldshaking development of atomic energy.” This I am indebted for counsel to Coit Blacker, McGeorge Bundy, Alexander Dallin, Peter Galison, Allen Greb, Jonathan Haslam, Gregg Herken, David Holloway, Gail Lapidus, Condoleezza Rice, David Rosenberg, Scott Sagan, Martin Sherwin, and Herbert York; for various sources to Roger Anders, Nancy Bressler, Jack Holl, Sally Marks, and William Tuttle; for support to the Ford Foundation Program in International Security, Barbara and Howard Holme, the Center for the History of Physics (American Institute of Physics), the Harry S. Truman Library Institute, and the Center for International Security and Arms Control; for access to the James Conant papers to Theodore Conant; and for early access to the Lewis L. -

Lawrence Berkeley Lab.O,R~Tory ' '- '·' R-.: I V L: UNIVERSITY of CALIFORNIA E!::Rkr-·Lt..Ltvrf:NCE I '-~Eflailr",..,.~ IJ
LBL-26560. C'_~ Lawrence Berkeley Lab.o,r~tory ' '- '·' r-.: I V L: UNIVERSITY OF CALIFORNIA E!::RKr-·Lt..ltVRf:NCE I_ '-~EfLAilr",..,.~ IJ Presented at the Symposium "Science Advice to the President: The First 200 Years," at the Annual Meeting of the American Association for the Advancement of Science, San Francisco, CA, January 18, 1989 Science Advice to the President: During and Immediately after World War II G.T. Seaborg January 1989 . .• t .. _- "l~ ~ ..... ~ .. Prepared for the U.S. Department of Energy under Contract Number DE-AC03-76SF00098. DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, .or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. ~ '. SCIENCE ADVICE TO THE PRESIDENT: DURING AND IMMEDIATELY AFTER WORLD WAR II Presented by Glenn T.