Winter 1985 Gems & Gemology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exceptional Works of Art 2017 PUSHKIN ANTIQUES – MAYFAIR –
Exceptional works of art 2017 PUSHKIN ANTIQUES – MAYFAIR – At Pushkin Antiques we specialise in unique statement Each item is professionally selected and inspected pieces of antique silver as well as branded luxury items, to ensure we can give our customers a guarantee of stylish interior articles and objects d’art. authenticity and the required peace of mind when buying from us. Since the inception of our company, we’ve been at the forefront of online sales for high end, quality antiques. Our retail gallery is located on the lower floor of the world Our presence on most major platforms has allowed us famous Grays Antiques Centre in the heart of Mayfair. to consistently connect exquisite pieces with the most discerning collectors and interior decorators from all over the world with particular focus on the demands of the markets from the Far East, the Americas, Europe & Russia. www.pushkinantiques.com [email protected] We aim to provide the highest quality in every department: rare hand crafted articles, accurate item descriptions (+44) 02085 544 300 to include the history and provenance of each item, an (+44) 07595 595 079 extensive photography report, as well as a smooth buying process thus facilitating an efficient and pleasant online Shop 111, Lower Ground Floor, Grays Antiques Market. experience. 58 Davies St, London. W1K 5AB, UK. ALEX PUSHKIN OLGA PUSHKINA DUMITRU TIRA Founder & Director Managing Director Photographer Contents 6 ENGLISH SILVER 42 CHINESE SILVER 56 JAPANESE SILVER 66 INDIAN SILVER 78 BURMESE SILVER 86 CONTINENTAL SILVER 100 FRENCH SILVER 108 GERMAN SILVER 118 RUSSIAN SILVER 132 OBJECTS OF VERTU English Silver The style and technique in manufacturing silver during Hester Bateman (1708-1794) was one of the greatest this era (over 100 years) changed radically, reflecting silversmiths operating in this style, she is the most the variations in taste, society, costumes, economic and renowned and appreciated female silversmith of all time. -

Zincite (Zn, Mn2+)O
Zincite (Zn, Mn2+)O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6mm. Crystals rare, typically pyramidal, hemimorphic, with large {0001}, to 2.5 cm, rarely curved; in broad cleavages, foliated, granular, compact, massive. Twinning: On {0001}, with composition plane {0001}. Physical Properties: Cleavage: {1010}, perfect; parting on {0001}, commonly distinct. Fracture: Conchoidal. Tenacity: Brittle. Hardness = 4 VHN = 205–221 (100 g load). D(meas.) = 5.66(2) D(calc.) = 5.6730 Rare pale yellow fluorescence under LW UV. Optical Properties: Translucent, transparent in thin fragments. Color: Yellow-orange to deep red, rarely yellow, green, colorless; deep red to yellow in transmitted light; light rose-brown in reflected light, with strong red to yellow internal reflections. Streak: Yellow-orange. Luster: Subadamantine to resinous. Optical Class: Uniaxial (+). ω = 2.013 = 2.029 R1–R2: (400) 13.0–13.6, (420) 12.8–13.2, (440) 12.6–12.8, (460) 12.3–12.6, (480) 12.1–12.4, (500) 12.0–12.2, (520) 11.8–12.1, (540) 11.8–12.0, (560) 11.7–11.9, (580) 11.6–11.8, (600) 11.4–11.7, (620) 11.3–11.6, (640) 11.2–11.5, (660) 11.1–11.4, (680) 11.0–11.2, (700) 11.0–11.2 Cell Data: Space Group: P 63mc (synthetic). a = 3.24992(5) c = 5.20658(8) Z = 2 X-ray Powder Pattern: Synthetic. 2.476 (100), 2.816 (71), 2.602 (56), 1.626 (40), 1.477 (35), 1.911 (29), 1.379 (28) Chemistry: (1) (2) SiO2 0.08 FeO 0.01 0.23 MnO 0.27 0.29 ZnO 99.63 98.88 Total 99.99 [99.40] (1) Sterling Hill, New Jersey, USA. -

Download the Scanned
American Mineralogist, Volume 70, pages 379-387, 1985 Ma_nganesehumites and leucophoenicitesfrom Franklin and Sterling- Hill' NewJersev: 'i"? andimplications ;ifi':il1,;;lfiil11"r's' Perr J. Dullx Department of Mineral Sciences Smithsonian lnstitution, Washington, D. C. 20560 Abstract The manganesehumites, (alleghanyite, manganhumite, and sonolite),together with some Mn-bearing samplesof the Mg-humites,and the related phasesleucophoenicite and jerry- gibbsite,from the orebodiesat Franklin and SterlingHill, New Jersey,are describedtogether with analytical data. Solid solution betweenhumite and manganhumiteis at least partially continuous. Expected Mn/Mg solid solutions between alleghanyiteand chondrodite, and betweensonolite and clinohumite, are discontinuous; they are interrupted by apparently orderedphases. In all cases,the possibleorderings involve Zn as well as Mn and Mg. There are no Mn end-membersof the manganesehumites at this locality. Manganeseis apparently restricted in leucophoenicite(5.42-6.63 Mn per 7 octahedral cations) and in jerrygibbsite (7.79-8.02Mn per 9 octahedralcations). Calcium is common to both leucophoeniciteand jerrygibbsite,but among the Mn-humites,only sonoliteaccepts appreciable Ca (0.65Ca per 9 octahedralcations). There is a "threshold" level ofzinc in all studiedsamples; this "threshold" levelis a constantfor leucophoenicite1-9.3 Znper 3 Si)and alleghanyite(-O.2Zn per 2 Si). No samplesof leucophoeniciteor jerrygibbsite were found to be Zn-ftee,suggesting either that Zn is required for their stability, or that these two phasesmight not be stable as end-members.Fluorine is present in all the Mn-humites and is proportional to the Mg- content,but is absentin leucophoeniciteand jerrygibbsite. Introduction humite speciesoccur there; the Mg-humites occur in the The magnesiumhumite species(norbergite, chondrodite, host Franklin Marble for the most part, and the Mn- humite, and clinohumite) have been well-studiedand re- humites in the orebodiesthemselves. -

Nomenclature of the Garnet Supergroup
American Mineralogist, Volume 98, pages 785–811, 2013 IMA REPORT Nomenclature of the garnet supergroup EDWARD S. GREW,1,* ANDREW J. LOCOCK,2 STUART J. MILLS,3,† IRINA O. GALUSKINA,4 EVGENY V. GALUSKIN,4 AND ULF HÅLENIUS5 1School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, U.S.A. 2Department of Earth and Atmospheric Sciences, University of Alberta, Edmonton, Alberta T6G 2E3, Canada 3Geosciences, Museum Victoria, GPO Box 666, Melbourne 3001, Victoria, Australia 4Faculty of Earth Sciences, Department of Geochemistry, Mineralogy and Petrography, University of Silesia, Będzińska 60, 41-200 Sosnowiec, Poland 5Swedish Museum of Natural History, Department of Mineralogy, P.O. Box 50 007, 104 05 Stockholm, Sweden ABSTRACT The garnet supergroup includes all minerals isostructural with garnet regardless of what elements occupy the four atomic sites, i.e., the supergroup includes several chemical classes. There are pres- ently 32 approved species, with an additional 5 possible species needing further study to be approved. The general formula for the garnet supergroup minerals is {X3}[Y2](Z3)ϕ12, where X, Y, and Z refer to dodecahedral, octahedral, and tetrahedral sites, respectively, and ϕ is O, OH, or F. Most garnets are cubic, space group Ia3d (no. 230), but two OH-bearing species (henritermierite and holtstamite) have tetragonal symmetry, space group, I41/acd (no. 142), and their X, Z, and ϕ sites are split into more symmetrically unique atomic positions. Total charge at the Z site and symmetry are criteria for distinguishing groups, whereas the dominant-constituent and dominant-valency rules are critical in identifying species. Twenty-nine species belong to one of five groups: the tetragonal henritermierite group and the isometric bitikleite, schorlomite, garnet, and berzeliite groups with a total charge at Z of 8 (silicate), 9 (oxide), 10 (silicate), 12 (silicate), and 15 (vanadate, arsenate), respectively. -

Leucophoenicite Mn (Sio4)3(OH)2
2+ Leucophoenicite Mn7 (SiO4)3(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals rare, typically slender, prismatic, elongated and striated [010], to 8 mm; in isolated grains or granular massive. Twinning: On k 001 , common, contact or interpenetrant twins, lamellar. f g Physical Properties: Cleavage: 001 , imperfect. Tenacity: Brittle. Hardness = 5.5{6 f g D(meas.) = 3.848 D(calc.) = [4.01] Optical Properties: Transparent to translucent. Color: Brown to light purple-red, raspberry-red, deep pink to light pink; rose-red to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: Faint; rose-red 001 ; colorless 001 . Orientation: k f g ? f g X 001 cleavage. Dispersion: r > v; slight. ® = 1.751(3) ¯ = 1.771(3) ° = 1.782(3) ? f g 2V(meas.) = 74(5)± Cell Data: Space Group: P 21=a: a = 10.842(19) b = 4.826(6) c = 11.324(9) ¯ = 103:93(9)± Z = [2] X-ray Powder Pattern: Franklin, New Jersey, USA. 1.8063 (10), 2.877 (9), 2.684 (8), 4.36 (5), 3.612 (5), 2.365 (5), 2.620 (4) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 26.36 26.7 26.7 CaO 5.67 2.4 2.8 FeO trace 0.3 0.3 Na2O 0.39 MnO 60.63 62.8 64.7 K2O 0.24 ZnO 3.87 0.0 0.0 H2O 2.64 [2.3] [2.8] MgO 0.21 5.5 2.7 Total 100.01 [100.0] [100.0] (1) Franklin, New Jersey, USA; composite of two analyses, corresponding to (Mn5:89Ca0:70Zn0:32 Na0:04Mg0:03K0:01)§=6:99(Si1:01O4)3(OH)2: (2) Kombat mine, Namibia; by electron microprobe, H2O by di®erence; corresponding to (Mn5:98Mg0:92Ca0:29Fe0:02)§=7:21(SiO4)3(OH)1:72: (3) Valsesia-Valtournanche area, Italy; by electron microprobe, H2O by di®erence; corresponding to (Mn6:16Mg0:45Ca0:34Fe0:03)§=6:98(SiO4)3(OH)2:10: Mineral Group: Leucophoenicite group. -

TEPHROITE from FRANKLIN, NEW JERSEY* Connbrrus S. Hunrsur
THE AMERICAN MINERALOGIST, VOL 46, MAY_JUNE, 1961 TEPHROITE FROM FRANKLIN, NEW JERSEY* ConNBrrus S. Hunrsur, Jn., Departmentof Mineralogy, Harvard, Uniaersity. Assrnlcr A study of tephroite specimens from Franklin and Sterling Hill, New Jersey showed in all of them the presence of thin sheets of willemite believed to be a product of exsolution. .fhese sheets are oriented parallel to the {100} and [010] planes of tephroite with the o and r axes of tephroite and willemite parallel. It is believed that Iittle zinc remains in the tephroite structure and that much of it reported in chemical analyses has been con- tributed by intergrown u'illemite. This conclusion is supported by experiments syn- thesizing tephroite. The indices of refraction and d spacing of {130} vary as would be expected with changes in amounts of MgO, FeO and CaO. INrnooucrroN 'fephroite, Mn2SiO4,a member of the olivine group, was describedas a new mineral from SterlingHill by Breithaupt in 1823.A chemicalanal- ysis of the original material was published by Brush (1864) together with severaladditional chemical analysesof tephroite made by others. These analysesreport ZnO in varying amounts which Brush attributed to invariably associatedzincite. Palache (1937) did not agree with Brush and stated " . that the molecularratios in someanalyses more nearly satisfy the orthosilicateformula when zinc is regardedas essen- tially a part of the mineral rather than as a constituent of mechanical inclusions." The present study was undertaken for the purpose of in- vestigatingthe variations in the propertiesof tephroite with changesin chemical composition, particularly the effect of zinc. Relationships were not expectedto be simplefor analysesshow, in addition to ZnO, variable amounts of MgO, FeO, and CaO. -

Tobias Okamoto Alm
THE TOOL BELT A Masculine Jewellery Practice Tobias Okamoto Alm Konstfack University College of Arts, Crafts and Design Jewellery + Corpus Master, Essay Spring 2014 Tutor: Christina Zetterlund ABSTRACT This essay is built upon the argument that the tool belt is a piece of jewellery and it investigates where and how the tool belt affects social dynamics in society. It explores the intersections between equipment, accessory, and adornment; where practical and social functionality coexist in a symbiosis. The text adopts a critical gender perspective on jewellery and masculinity; it examines the strategies employed in masculine environments in order to be able to wear jewellery without the risk of feminization. It describes a process of myth creation, surrounding an idealised masculinity built upon rationality and resourcefulness, glorifying the professional construction worker. Acknowledging the tool belt as a piece of jewellery diversifies and enriches the jewellery field. It also expands the perspective on jewellery and masculinity, while opening up for further investigation of the relationship between adornment and gender dynamics. 1 TABLE OF CONTENTS ABSTRACT ............................................................................................................................................................................................................ 1 JEWELLERY IN SOCIETY ................................................................................................................................................................................. -
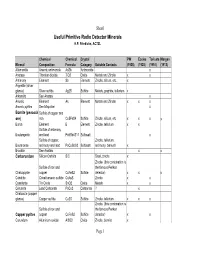
Useful Primitive Radio Detector Minerals H.P
Sheet1 Useful Primitive Radio Detector Minerals H.P. Friedichs, AC7ZL Chemical Chemical Crystal PW Eccles Toricata Morgan Mineral Composition Formula Category Suitable Contacts (1925) (1928) (1910) (1913) Allemontite Arsenic antimonide AsSb Antimonide x Anatase Titanium dioxide TiO2 Oxide Metals and Zincite x x Antimony Element Sb Element Zincite, silicon, etc. x Argentite (silver glance) Silver sulfide Ag2S Sulfide Metals, graphite, tellurium x Arkansite See Anatase x Arsenic Element As Element Metals and Zincite x x x Arsenic pyrites See Mispickel x Bornite (peacock Sulfide of copper and ore) iron Cu5FeS4 Sulfide Zincite, silicon, etc. x x x x Boron Element B Element Zincite, tellurium x x Sulfide of antimony Boulangerite and lead Pb5Sb4S11 Sulfosalt x Sulfide of copper, Zincite, tellurium, Bourmonite antimony and lead PbCuSbS3 Sulfosalt antimony, bismuth x Brookite See Anatase x x Carborundum Silicon Carbide SiC Steel, zincite x Zincite (this combination is Sulfide of iron and the famous Perikon Chalcopyrite copper CuFeS2 Sulfide detector) x x x Cobaltite Cobalt arsenic sulfide CoAsS Zincite x x Cassiterite Tin Oxide SnO2 Oxide Metals x x Cerussite Lead Carbonate PbCo3 Carbonate ?x Chalcocite (copper glance) Copper sulfide Cu2S Sulfide Zincite, tellurium x x x Zincite (this combination is Sulfide of iron and the famous Perikon Copper pyrites copper CuFeS2 Sulfide detector) x x Corundum Aluminum oxidde Al2O3 Oxide Zincite, bornite x Page 1 Sheet1 Covellite Copper sulfide CuS Sulfide Zincite, etc x x Cuprite (Cuprous oxide) Copper oxide -

Aire Currents
Aire Currents Newsletter for the Canton of Aire Faucon City, NC 28016 http://aire.atlantia.sca.org/airenoframe.htm Volume VII, Issues I- VI January- June A.S. XXXVIII Janet Thompson 515 E. Ohio Avenue Bessemer Come visit us on the web… being 2004 Gregorian Canton Regnum for Aire Faucon Seneschal & Chronicler: Deputy Seneschale: Lady Maeve Griffinsward Lady Brianna O’Duinn (Janet Thompson) (Sherra Dunn) [email protected] [email protected] Chatelaine: OPEN MoAS & Deputy Chronicler: Lady Katerina Sina Samovicha Kathryn Evans [email protected] Exchequer: Herald: Lord Otto von Schwyz OPEN (Tim White) [email protected] HAPPY BIRTHDAY TO: Webminister: Knight’s Marshal: Lord Cyriac Grymsdale Baroness Fiona MacLeod Jessica August 7 (Kevin C Towery) (Andrea Davis) [email protected] [email protected] Johan August 24 Fiona (Megan) August 28 MEETING MINUTES January 13, 2004 New Business: Ymir – remember to get reservations in. Inn on the Road - $352 total expense for feast - $90 people cooking (360) - $20-$25 children – Caterine St. Loe will do children’s’ activities. $? Prizes for tourneys Donated. Making decorations, bring banners, count on 90 people, and encourage reservations, charity to be the battered women’s shelter. Novice Tourney- Challenge of Phoenix: Gaelan to use Robear’s field kitchen. Working on a bid for IAPE for January at Daniel Stowe Gardens Seneschal: 4th quarter reports for seneschal, chronicler, MoM & Waivers are in. Rapier charity tourney @ Ymir – will get name of charity for checks – collect practice, university & Feb meetings @ Friday night of Ymir – New web minister – Kevin (Cyriac) Exchequer: getting married, will do report ASAP. $1243.94 in acct. -
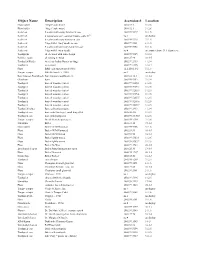
Object Name Description Accession # Location
Object Name Description Accession # Location Floss holder “finger” style metal 2010.12.1 31.2.6 Floss holder “finger” style wood 2010.12.1 31.2.6 Scaler set 5 scalers with ebony handles in case 2002.99.5327 31.1.5 Scaler set 6 instruments with common handle, early 19th no # on display Scaler set 6 scalers with ivory handles in case 2002.99.5328 31.1.5 Scaler set 7 tips with 1 ivory handle in case 2002.99.5331 31.1.5 Scaler set 9 scalers with one ivory handle in case 2002.99.5358 31.1.6 Scaler set 9 tips with 1 ivory handle no # on counter above 31.1 drawer set Toothpicks all are wood with same design 2002.99.5315 31.2.4 Polisher, tooth all writing in Asian 2003.27.96 16-1-5 Toothpick Holder American Indian Hunter on Stage 2002.99.2933 11.3.4 Toothpick as a sword 2002.99.5136 31.2.4 Floss B&B early tin (patented 1906) 12.2.250.1.1-2 31.1.6 Tongue scraper Bakelite handle c. 1920 no # on display Bart Simpson Toothbrush Bart Simpson toothbrush set 2003.8.132.1 18-1-2 Chatelaine bone 2002.99.5152 31.2.4 Toothpick bone & wooden (varies) 2002.99.5205.1 31.2.5 Toothpick bone & wooden (varies) 2002.99.5205.2 31.2.5 Toothpick bone & wooden (varies) 2002.99.5205.3 31.2.5 Toothpick bone & wooden (varies) 2002.99.5205.4 31.2.5 Toothpick bone & wooden (varies) 2002.99.5205.5 31.2.5 Toothpick bone & wooden (varies) 2002.99.5205.6 31.2.5 Toothpick bone & wooden (varies) 2002.99.5205.7 31.2.5 Toothpick holder Bone or Ivory porcupine 2002.99.2913 11.3.4 Toothpick case bone with Dutch girl, small hinged lid 2005.10.215 31.2.3 Toothpick case bone with toothpicks -

Vintage Jewellery & Accessories
Vintage Jewellery & Accessories Monday 18th July 2016 VintageVintage Jewellery Jewellery & & Accessories Accessories MondayMonday 18th25th JulyJanuary 2016 2016at 10am at 10am With over a thousand lots in this auction we should be able to satisfy the needs of all our buyers. EnamelFor our fijewellery rst Vintage is Jewelleryalways popular & Accessories and there auction are some of 2016 beautiful we have early 20thbrooch century and pieces, ear clips including by Nanna lot Ditzel 102, foran Georgenamel Jensen and marcasite are bound to Bernardsome great Instone items floral for you. necklace, Whether if you you are are looking a fan forof hisValentine’s work we gifts also or have bean a eyehit withcatching buyers. Bernard The most Instone striking ring, piece lot 103.I feel is lot 571, an 18ct just something to add to your own collection, we can cover it. gold lapis lazuli and diamond ring by Lapponia, although it’s an abstract We have a great selection of coral jewellery again in this auction. Lot 259 designis bound it’s to very prove elegant. very popular, a mid 19th century figa pendant, thisThere design are some is always beautiful sort pieces after. of Scottish jewellery, for example lot 181, a late Victorian gold Scottish agate set brooch. There are also Antique jewellery cases have been selling very well in the last two There is a wide selection of amber jewellery, with the ever popular Baltic amber bead necklaces and also a great selection of Burmese banded agate bead necklaces, these always prove popular so should auctions and we have lots more in this auction, from lot 742. -

Mineral Minutes May 2017 A.Pdf
THE MONTHLY NEWSLETTER OF THE MINERALOGICAL SOCIETY OF THE DISTRICT OF COLUMBIA The Mineral Volume 75-05 In this Issue: May 2017 The Prez Says⁄ Page 1 i May Program – „Geology in the National Capital Region‰ Page 1 Minutes of the April Business Meeting Page 4 April 2017 Program „What's New in Smithsonian Gems Prez Page 4 Minerals‰ Says... n Geologists Uncover Three New Uranyl Minerals Page 6 Large, Exceptional Gem Diamonds Formed from Metallic Page 7 by Dave Liquid inside EarthÊs Mantle Nanney, The Earth's Crust Is Getting Thinner Than Ever Before Page 8 MSDC President u Evidence of Ancient 'Geological Brexit' Revealed Page 9 Sapphire Rush Near Ambatondrazaka, Madagascar Page 10 A Photo-Tour of Garnets...Now in Every Color! Page 11 Blue Dravite-Uvite Tourmaline from Koksha Valley, pring is finally here. It was just Page 16 t Afghanistan pretending for the first couple of Mineral of the Month - Platinum Page 18 Sweeks but by the first week in April, Minerals of Ireland Page 19 IT IS HERE. Our garden is getting really pretty as about a third of our plants are e Geologist of the Month – John Sinkankas Page 20 in bloom. Please join us on 30 April from In Memorium - George Reimherr Page 22 1-5PM for our open house where you Mineralogical Society of America EditorsÊ Picks Page 23 can see our mineral collection, wander Upcoming Geology Events Page 24 through our 2 ½ acre garden, while carrying fluids of your choice. Reach out s Useful Mineral Links Page 24 and we can send you directions.