Structural Organization of Trypanosoma Cruzi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download the Abstract Book
1 Exploring the male-induced female reproduction of Schistosoma mansoni in a novel medium Jipeng Wang1, Rui Chen1, James Collins1 1) UT Southwestern Medical Center. Schistosomiasis is a neglected tropical disease caused by schistosome parasites that infect over 200 million people. The prodigious egg output of these parasites is the sole driver of pathology due to infection. Female schistosomes rely on continuous pairing with male worms to fuel the maturation of their reproductive organs, yet our understanding of their sexual reproduction is limited because egg production is not sustained for more than a few days in vitro. Here, we explore the process of male-stimulated female maturation in our newly developed ABC169 medium and demonstrate that physical contact with a male worm, and not insemination, is sufficient to induce female development and the production of viable parthenogenetic haploid embryos. By performing an RNAi screen for genes whose expression was enriched in the female reproductive organs, we identify a single nuclear hormone receptor that is required for differentiation and maturation of germ line stem cells in female gonad. Furthermore, we screen genes in non-reproductive tissues that maybe involved in mediating cell signaling during the male-female interplay and identify a transcription factor gli1 whose knockdown prevents male worms from inducing the female sexual maturation while having no effect on male:female pairing. Using RNA-seq, we characterize the gene expression changes of male worms after gli1 knockdown as well as the female transcriptomic changes after pairing with gli1-knockdown males. We are currently exploring the downstream genes of this transcription factor that may mediate the male stimulus associated with pairing. -

Autophagy in Trypanosomatids
Cells 2012, 1, 346-371; doi:10.3390/cells1030346 OPEN ACCESS cells ISSN 2073-4409 www.mdpi.com/journal/cells Review Autophagy in Trypanosomatids Ana Brennand 1,†, Eva Rico 2,†,‡ and Paul A. M. Michels 1,* 1 Research Unit for Tropical Diseases, de Duve Institute, Université catholique de Louvain, Avenue Hippocrate 74, postal box B1.74.01, B-1200 Brussels, Belgium; E-Mail: [email protected] 2 Department of Biochemistry and Molecular Biology, University Campus, University of Alcalá, Alcalá de Henares, Madrid, 28871, Spain; E-Mail: [email protected] † These authors contributed equally to this work. ‡ Present Address: Centre for Immunity, Infection and Evolution, Institute of Immunology and Infection Research, School of Biological Sciences, King’s Buildings, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-2-7647473; Fax: +32-2-7626853. Received: 28 June 2012; in revised form: 14 July 2012 / Accepted: 16 July 2012 / Published: 27 July 2012 Abstract: Autophagy is a ubiquitous eukaryotic process that also occurs in trypanosomatid parasites, protist organisms belonging to the supergroup Excavata, distinct from the supergroup Opistokontha that includes mammals and fungi. Half of the known yeast and mammalian AuTophaGy (ATG) proteins were detected in trypanosomatids, although with low sequence conservation. Trypanosomatids such as Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. are responsible for serious tropical diseases in humans. The parasites are transmitted by insects and, consequently, have a complicated life cycle during which they undergo dramatic morphological and metabolic transformations to adapt to the different environments. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma Brucei in the Mammalian Bloodstream
pathogens Review The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma brucei in the Mammalian Bloodstream Eleanor Silvester †, Kirsty R. McWilliam † and Keith R. Matthews * Institute for Immunology and Infection Research, Centre for Immunity, Infection and Evolution, School of Biological Sciences, King’s Buildings, University of Edinburgh, Charlotte Auerbach Road, Edinburgh EH9 3FL, UK; [email protected] (E.S.); [email protected] (K.R.McW.) * Correspondence: [email protected]; Tel.: +44-131-651-3639 † These authors contributed equally to this work. Received: 23 May 2017; Accepted: 22 June 2017; Published: 28 June 2017 Abstract: African trypanosomes cause devastating disease in sub-Saharan Africa in humans and livestock. The parasite lives extracellularly within the bloodstream of mammalian hosts and is transmitted by blood-feeding tsetse flies. In the blood, trypanosomes exhibit two developmental forms: the slender form and the stumpy form. The slender form proliferates in the bloodstream, establishes the parasite numbers and avoids host immunity through antigenic variation. The stumpy form, in contrast, is non-proliferative and is adapted for transmission. Here, we overview the features of slender and stumpy form parasites in terms of their cytological and molecular characteristics and discuss how these contribute to their distinct biological functions. Thereafter, we describe the technical developments that have enabled recent discoveries that uncover how the slender to stumpy transition is enacted in molecular terms. Finally, we highlight new understanding of how control of the balance between slender and stumpy form parasites interfaces with other components of the infection dynamic of trypanosomes in their mammalian hosts. -

The Life Cycle of Trypanosoma Cruzi
Tyler et al. THE LIFE CYCLE OF TRYPANOSOMA CRUZI K. M. Tyler, C. L. Olson and D. M. Engman Departments of Microbiology-Immunology and Pathology Feinberg Medical School of Northwestern University, Chicago, IL 60611 ABSTRACT Since the discovery of Trypanosoma cruzi as the parasite that causes Chagas disease, nearly a century ago, the details of the organism's life cycle have fascinated scientists. T. cruzi is a single-celled eukaryote with a complex life cycle alternating between reduviid bug vectors and vertebrate hosts. It is able to adapt via the process of cellular differentiation to replicate within the diverse environments represented of the insect's gut and host cell cytoplasm. These adaptive transformations take the form of coordinated changes in morphology, metabolism and cell cycle regulation. Different life cycle stages of T. cruzi show dramatically different protein and RNA profiles, which are the end result of unusual mechanisms for regulating gene expression. In recent years, new molecular techniques have been brought to bear on the life cycle dramatically increasing our knowledge of the strategies employed by the parasite to ensure its continued survival. INTRODUCTION Chagas disease The etiologic agent of the chronic and often fatal Chagas disease is the American trypanosome, Trypanosoma cruzi , a flagellated protozoan of the order Kinetoplastida. The survival of T. cruzi is dependent on the successful transmission between, and the colonization of, two radically different environments: the midgut of the reduviid bug vector and the cytoplasm of the mammalian host cell. As is true of all infections, interruption of the pathogen's life cycle will lead to eradication of the disease. -

A PRELIMINARY NOTE on TETRAMITUS, a STAGE in the LIFE CYCLE of a COPROZOIC AMOEBA by MARTHA BUNTING
294 Z6OL6G Y.- M. BUNTING PROC. N. A. S. A PRELIMINARY NOTE ON TETRAMITUS, A STAGE IN THE LIFE CYCLE OF A COPROZOIC AMOEBA By MARTHA BUNTING DEPARTMENT OF ZOOLOGY, UNIVERSITY OF PENNSYLVANIA Communicated July 24, 1922 In 1920 a study of the Entamoeba of the rat was begun and in a culture on artificial medium a number of Coprozoic amoeba appeared, one of which exhibited a flagellate phase which seems to be identical with Tetramitus rostratus Perty.1 The appearance of flagellate phases in cultures of Coprozoic amoeba (Whitmore,2 etc.) as well as of soil amoeba (Kofoid,1 Wilson,4 etc.) has been recorded a number of times, but the flagellates which appeared were relatively simple in organization and very transitory in occurrence. Fur- thermore, some of the simpler flagellates have been observed (Pascher5) to lose their flagella and become amoeboid. In the case here described, however, there is a transformation of a simple amoeba into a relatively complex flagellate which multiplies for several days then returns to the amoeboid condition and becomes encysted. Tetramitus rostratus has been studied by a number of investigators since its discovery in 1852, by Perty,' yet no one has given a full account of its life history. The lack of cysts in previous accounts, mentioned by Dobell and O'Connor,6 is explained by the transformation into an amoeba before encystment. It is believed that this animal presents the extreme in transformations of this sort and serves to emphasize the close relationship between amoebae and flagellates, and the need for careful studies of life cycles, in pure cultures where possible, in investigations of coprozoic and other Protozoa. -

Kmcb-Abstract-Book-2019-Final
VIII April 27 – May 1, 2019 2 Eight Kinetoplastid Molecular Cell Biology Meeting April 27 – May 1, 2019 Hosted by the Marine Biological Laboratory Woods Hole, Massachusetts, USA The meeting was founded by George A.M. Cross in 2005 Organizers George A.M. Cross 2005-2011 Christian Tschudi 2013-2019 3 KMCBM 2019 Acknowledgements The organizer wishes to thank: The Program Committee Barbara Burleigh (Harvard T. H. Chan School of Public Health, Boston, USA) James D. Bangs (University at Buffalo, Buffalo, USA) Stephen M. Beverley (Washington University School of Medicine, St. Louis, USA) Keith R. Matthews (The University of Edinburgh, Edinburgh, Scotland, UK) The Staff at MBL: Paul Anderson and the MBL Housing and Conference Staff for registration and housing; All the IT AV Support staff and the staff in Sodexo Food Service at the MBL. Cover Design: Markus Engstler 4 KMCBM 2019 Program Saturday, April 27 02:00 – 05:00 Arrival, Registration and Poster Session A setup 04:00 – 06:30 Greeting and Dinner 07:00 – 09:00 Session I: VSG (chair: Mark Carrington) 09:00 – 11:00 Mixer Sunday, April 28 07:00 – 08:30 Breakfast 08:45 – 11:45 Session II: Biochemistry/Metabolism (chair: Ken Stuart) 12:00 – 01:30 Lunch 02:00 – 04:30 Session III: Cell Biology (chair: Kimberly Paul) 06:00 – 07:00 Dinner 07:00 – 09:00 POSTER PRESENTATIONS: Session A 09:00 – 11:00 Mixer & Poster A/B Changeover Monday, April 29 07:00 – 08:30 Breakfast 08:45 – 11:45 Session IV: Pathogenesis I (chair: Luisa Figueiredo) 12:00 – 01:30 Lunch 01:30 – 06:00 Free Time 06:00 – 07:00 Dinner 07:00 -

Cell & Molecular Biology
BSC ZO- 102 B. Sc. I YEAR CELL & MOLECULAR BIOLOGY DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY BSCZO-102 Cell and Molecular Biology DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY Phone No. 05946-261122, 261123 Toll free No. 18001804025 Fax No. 05946-264232, E. mail [email protected] htpp://uou.ac.in Board of Studies and Programme Coordinator Board of Studies Prof. B.D.Joshi Prof. H.C.S.Bisht Retd.Prof. Department of Zoology Department of Zoology DSB Campus, Kumaun University, Gurukul Kangri, University Nainital Haridwar Prof. H.C.Tiwari Dr.N.N.Pandey Retd. Prof. & Principal Senior Scientist, Department of Zoology, Directorate of Coldwater Fisheries MB Govt.PG College (ICAR) Haldwani Nainital. Bhimtal (Nainital). Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Programme Coordinator Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Haldwani, Nainital Unit writing and Editing Editor Writer Dr.(Ms) Meenu Vats Dr.Mamtesh Kumari , Professor & Head Associate. Professor Department of Zoology, Department of Zoology DAV College,Sector-10 Govt. PG College Chandigarh-160011 Uttarkashi (Uttarakhand) Dr.Sunil Bhandari Asstt. Professor. Department of Zoology BGR Campus Pauri, HNB (Central University) Garhwal. Course Title and Code : Cell and Molecular Biology (BSCZO 102) ISBN : 978-93-85740-54-1 Copyright : Uttarakhand Open University Edition : 2017 Published By : Uttarakhand Open University, Haldwani, Nainital- 263139 Contents Course 1: Cell and Molecular Biology Course code: BSCZO102 Credit: 3 Unit Block and Unit title Page number Number Block 1 Cell Biology or Cytology 1-128 1 Cell Type : History and origin. -

A Monograph on the Protozoa of the Large Intestine of the Horse Ta-Shih Hsiung Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1930 A monograph on the protozoa of the large intestine of the horse Ta-Shih Hsiung Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Zoology Commons Recommended Citation Hsiung, Ta-Shih, "A monograph on the protozoa of the large intestine of the horse" (1930). Retrospective Theses and Dissertations. 14768. https://lib.dr.iastate.edu/rtd/14768 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overiaps. -

The Flagellum and Flagellar Pocket of Trypanosomatids
Molecular & Biochemical Parasitology 115 (2001) 1–17 www.parasitology-online.com. Reviews: Parasite cell Biology: 1 The flagellum and flagellar pocket of trypanosomatids Scott M. Landfear *, Marina Ignatushchenko Department of Molecular Microbiology and Immunology, Oregon Health Sciences Uni6ersity, Portland, OR 97201, USA Received 9 November 2000; received in revised form 26 January 2001; accepted 5 March 2001 Abstract The flagellum and flagellar pocket are distinctive organelles present among all of the trypanosomatid protozoa. Currently, recognized functions for these organelles include generation of motility for the flagellum and dedicated secretory and endocytic activities for the flagellar pocket. The flagellar and flagellar pocket membranes have long been recognized as morphologically separate domains that are component parts of the plasma membrane that surrounds the entire cell. The structural and functional specialization of these two membranes has now been underscored by the identification of multiple proteins that are targeted selectively to each of these domains, and non-membrane proteins have also been identified that are targeted to the internal lumina of these organelles. Investigations on the functions of these organelle-specific proteins should continue to shed light on the unique biological activities of the flagellum and flagellar pocket. In addition, work has begun on identifying signals or modifications of these proteins that direct their targeting to the correct subcellular location. Future endeavors should further refine our knowledge of targeting signals and begin to dissect the molecular machinery involved in transporting and retaining each polypeptide at its designated cellular address. © 2001 Elsevier Science B.V. All rights reserved. Keywords: Trypanosomatid protozoa; Flagellum; Flagellar Pocket; Organelle-specific proteins; Review 1. -
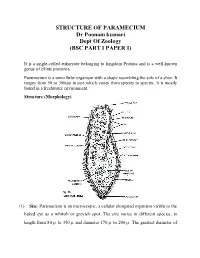
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

Glycosome Biogenesis in Trypanosomes and the De Novo Dilemma
REVIEW Glycosome biogenesis in trypanosomes and the de novo dilemma Sarah Bauer, Meredith T. Morris* Eukaryotic Pathogens Innovation Center, Department of Genetics and Biochemistry, Clemson University, Clemson, South Carolina, United States of America * [email protected] Abstract Trypanosomatid parasites, including Trypanosoma and Leishmania, are the causative a1111111111 agents of lethal diseases threatening millions of people around the world. These organisms a1111111111 compartmentalize glycolysis in essential, specialized peroxisomes called glycosomes. Per- a1111111111 oxisome proliferation can occur through growth and division of existing organelles and de a1111111111 a1111111111 novo biogenesis from the endoplasmic reticulum. The level that each pathway contributes is debated. Current evidence supports the concerted contribution of both mechanisms in an equilibrium that can vary depending on environmental conditions and metabolic require- ments of the cell. Homologs of a number of peroxins, the proteins involved in peroxisome biogenesis and matrix protein import, have been identified in T. brucei. Based on these find- OPEN ACCESS ings, it is widely accepted that glycosomes proliferate through growth and division of existing Citation: Bauer S, Morris MT (2017) Glycosome organelles; however, to our knowledge, a de novo mechanism of biogenesis has not been biogenesis in trypanosomes and the de novo dilemma. PLoS Negl Trop Dis 11(4): e0005333. directly demonstrated. Here, we review recent findings that provide support for the existence https://doi.org/10.1371/journal.pntd.0005333 of an endoplasmic reticulum (ER)-derived de novo pathway of glycosome biogenesis in T. Editor: Christian Tschudi, Yale School of Public brucei. Two studies recently identified PEX13.1, a peroxin involved in matrix protein import, Health, UNITED STATES in the ER of procyclic form T.