3Rd Annual Student Research Symposium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download the Abstract Book
1 Exploring the male-induced female reproduction of Schistosoma mansoni in a novel medium Jipeng Wang1, Rui Chen1, James Collins1 1) UT Southwestern Medical Center. Schistosomiasis is a neglected tropical disease caused by schistosome parasites that infect over 200 million people. The prodigious egg output of these parasites is the sole driver of pathology due to infection. Female schistosomes rely on continuous pairing with male worms to fuel the maturation of their reproductive organs, yet our understanding of their sexual reproduction is limited because egg production is not sustained for more than a few days in vitro. Here, we explore the process of male-stimulated female maturation in our newly developed ABC169 medium and demonstrate that physical contact with a male worm, and not insemination, is sufficient to induce female development and the production of viable parthenogenetic haploid embryos. By performing an RNAi screen for genes whose expression was enriched in the female reproductive organs, we identify a single nuclear hormone receptor that is required for differentiation and maturation of germ line stem cells in female gonad. Furthermore, we screen genes in non-reproductive tissues that maybe involved in mediating cell signaling during the male-female interplay and identify a transcription factor gli1 whose knockdown prevents male worms from inducing the female sexual maturation while having no effect on male:female pairing. Using RNA-seq, we characterize the gene expression changes of male worms after gli1 knockdown as well as the female transcriptomic changes after pairing with gli1-knockdown males. We are currently exploring the downstream genes of this transcription factor that may mediate the male stimulus associated with pairing. -

Autophagy in Trypanosomatids
Cells 2012, 1, 346-371; doi:10.3390/cells1030346 OPEN ACCESS cells ISSN 2073-4409 www.mdpi.com/journal/cells Review Autophagy in Trypanosomatids Ana Brennand 1,†, Eva Rico 2,†,‡ and Paul A. M. Michels 1,* 1 Research Unit for Tropical Diseases, de Duve Institute, Université catholique de Louvain, Avenue Hippocrate 74, postal box B1.74.01, B-1200 Brussels, Belgium; E-Mail: [email protected] 2 Department of Biochemistry and Molecular Biology, University Campus, University of Alcalá, Alcalá de Henares, Madrid, 28871, Spain; E-Mail: [email protected] † These authors contributed equally to this work. ‡ Present Address: Centre for Immunity, Infection and Evolution, Institute of Immunology and Infection Research, School of Biological Sciences, King’s Buildings, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-2-7647473; Fax: +32-2-7626853. Received: 28 June 2012; in revised form: 14 July 2012 / Accepted: 16 July 2012 / Published: 27 July 2012 Abstract: Autophagy is a ubiquitous eukaryotic process that also occurs in trypanosomatid parasites, protist organisms belonging to the supergroup Excavata, distinct from the supergroup Opistokontha that includes mammals and fungi. Half of the known yeast and mammalian AuTophaGy (ATG) proteins were detected in trypanosomatids, although with low sequence conservation. Trypanosomatids such as Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. are responsible for serious tropical diseases in humans. The parasites are transmitted by insects and, consequently, have a complicated life cycle during which they undergo dramatic morphological and metabolic transformations to adapt to the different environments. -

Mrs. Stager- English 4 - All Sections
2020 Summer Assignments - Mrs. Stager- English 4 - all sections English 4 Summer Reading: Circe by Madeline Miller; available in paperback, e-book and audio book. The study guide (see link below ) is due by the first day of class and will be used to write an essay during the first few weeks of school. The novel will be used in the first unit of study in Eng 4. Link to info on the book and the study guide: https://docs.google.com/document/d/1kXsSizZNNT9eqlMFTSD7nmIVUiuiftqGgp9r7fGs5d0/edit?usp=sharing AND Every student in English 4 is required to write a first draft college application essay and share it with Mrs. Stager on Google Docs by the first day of school. Instructions: ● Select a Common App essay topic listed below OR you may write based on a prompt for a specific school to which you are applying. **NOTE: If you are writing an essay for a specific college, you must include the entire essay prompt. This will allow me to ensure you are addressing the essay prompt adequately. ● Review the rubric for complete instructions; a large part of your grade for this 1st draft is following the instructions. ● Keep in mind that this is your first draft, and we will revise once school begins; sometimes getting started is the most difficult part of the process (see below for essay tips). ● If you need a finalized essay for an application before school starts, please let me know. I will check my email a few times a week throughout the summer. DO NOT WRITE YOUR ESSAY ABOUT COVID - If you write about COVID, will ask you to re-write it. -

The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma Brucei in the Mammalian Bloodstream
pathogens Review The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma brucei in the Mammalian Bloodstream Eleanor Silvester †, Kirsty R. McWilliam † and Keith R. Matthews * Institute for Immunology and Infection Research, Centre for Immunity, Infection and Evolution, School of Biological Sciences, King’s Buildings, University of Edinburgh, Charlotte Auerbach Road, Edinburgh EH9 3FL, UK; [email protected] (E.S.); [email protected] (K.R.McW.) * Correspondence: [email protected]; Tel.: +44-131-651-3639 † These authors contributed equally to this work. Received: 23 May 2017; Accepted: 22 June 2017; Published: 28 June 2017 Abstract: African trypanosomes cause devastating disease in sub-Saharan Africa in humans and livestock. The parasite lives extracellularly within the bloodstream of mammalian hosts and is transmitted by blood-feeding tsetse flies. In the blood, trypanosomes exhibit two developmental forms: the slender form and the stumpy form. The slender form proliferates in the bloodstream, establishes the parasite numbers and avoids host immunity through antigenic variation. The stumpy form, in contrast, is non-proliferative and is adapted for transmission. Here, we overview the features of slender and stumpy form parasites in terms of their cytological and molecular characteristics and discuss how these contribute to their distinct biological functions. Thereafter, we describe the technical developments that have enabled recent discoveries that uncover how the slender to stumpy transition is enacted in molecular terms. Finally, we highlight new understanding of how control of the balance between slender and stumpy form parasites interfaces with other components of the infection dynamic of trypanosomes in their mammalian hosts. -

Kmcb-Abstract-Book-2019-Final
VIII April 27 – May 1, 2019 2 Eight Kinetoplastid Molecular Cell Biology Meeting April 27 – May 1, 2019 Hosted by the Marine Biological Laboratory Woods Hole, Massachusetts, USA The meeting was founded by George A.M. Cross in 2005 Organizers George A.M. Cross 2005-2011 Christian Tschudi 2013-2019 3 KMCBM 2019 Acknowledgements The organizer wishes to thank: The Program Committee Barbara Burleigh (Harvard T. H. Chan School of Public Health, Boston, USA) James D. Bangs (University at Buffalo, Buffalo, USA) Stephen M. Beverley (Washington University School of Medicine, St. Louis, USA) Keith R. Matthews (The University of Edinburgh, Edinburgh, Scotland, UK) The Staff at MBL: Paul Anderson and the MBL Housing and Conference Staff for registration and housing; All the IT AV Support staff and the staff in Sodexo Food Service at the MBL. Cover Design: Markus Engstler 4 KMCBM 2019 Program Saturday, April 27 02:00 – 05:00 Arrival, Registration and Poster Session A setup 04:00 – 06:30 Greeting and Dinner 07:00 – 09:00 Session I: VSG (chair: Mark Carrington) 09:00 – 11:00 Mixer Sunday, April 28 07:00 – 08:30 Breakfast 08:45 – 11:45 Session II: Biochemistry/Metabolism (chair: Ken Stuart) 12:00 – 01:30 Lunch 02:00 – 04:30 Session III: Cell Biology (chair: Kimberly Paul) 06:00 – 07:00 Dinner 07:00 – 09:00 POSTER PRESENTATIONS: Session A 09:00 – 11:00 Mixer & Poster A/B Changeover Monday, April 29 07:00 – 08:30 Breakfast 08:45 – 11:45 Session IV: Pathogenesis I (chair: Luisa Figueiredo) 12:00 – 01:30 Lunch 01:30 – 06:00 Free Time 06:00 – 07:00 Dinner 07:00 -

Madelines Rescue Pdf, Epub, Ebook
MADELINES RESCUE PDF, EPUB, EBOOK Bemelmans Ludwig | 64 pages | 22 Nov 2007 | Penguin Putnam Inc | 9780140566512 | English | New York, NY, United States Madelines Rescue PDF Book He is most noted today for his Madeline books, six of which were published from Video Audio icon An illustration of an audio speaker. The first drawing of the girls fighting over who would get to sleep with the dog; Madeline looking out the window onto darkened Paris streets wishing for the dog Genevieve to return; and the entire sequence of the girls and Ms. More filters. Miss Clavel shines here, taking care of the girls' needs ahead of the commands of the out of touch trustees. I One of the few Caldecott winners that I actually read quite a bit as a child. Clavel quickly defuses it by announcing that should there be another fight over Genevieve, she will be given away. Download for print-disabled. So for that, 3 stars. Advanced Search Links. The pages with paintings are very well done, but the line art on the alternating pages looks sloppy to me. Help Learn to edit Community portal Recent changes Upload file. Ludwig Bemelmans was a painter, illustrator, and writer for both children and adults. But all is not well for Miss Clavel, since the twelve little girls all want a dog like Genevieve. In the original special, Madeline's Rescue , Cucuface steals Genevieve causing Miss Clavel and the girls to look for her in vain. Madeline's Rescue is a children's picture book by Ludwig Bemelmans , the second in the Madeline series. -

Cell & Molecular Biology
BSC ZO- 102 B. Sc. I YEAR CELL & MOLECULAR BIOLOGY DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY BSCZO-102 Cell and Molecular Biology DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY Phone No. 05946-261122, 261123 Toll free No. 18001804025 Fax No. 05946-264232, E. mail [email protected] htpp://uou.ac.in Board of Studies and Programme Coordinator Board of Studies Prof. B.D.Joshi Prof. H.C.S.Bisht Retd.Prof. Department of Zoology Department of Zoology DSB Campus, Kumaun University, Gurukul Kangri, University Nainital Haridwar Prof. H.C.Tiwari Dr.N.N.Pandey Retd. Prof. & Principal Senior Scientist, Department of Zoology, Directorate of Coldwater Fisheries MB Govt.PG College (ICAR) Haldwani Nainital. Bhimtal (Nainital). Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Programme Coordinator Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Haldwani, Nainital Unit writing and Editing Editor Writer Dr.(Ms) Meenu Vats Dr.Mamtesh Kumari , Professor & Head Associate. Professor Department of Zoology, Department of Zoology DAV College,Sector-10 Govt. PG College Chandigarh-160011 Uttarkashi (Uttarakhand) Dr.Sunil Bhandari Asstt. Professor. Department of Zoology BGR Campus Pauri, HNB (Central University) Garhwal. Course Title and Code : Cell and Molecular Biology (BSCZO 102) ISBN : 978-93-85740-54-1 Copyright : Uttarakhand Open University Edition : 2017 Published By : Uttarakhand Open University, Haldwani, Nainital- 263139 Contents Course 1: Cell and Molecular Biology Course code: BSCZO102 Credit: 3 Unit Block and Unit title Page number Number Block 1 Cell Biology or Cytology 1-128 1 Cell Type : History and origin. -
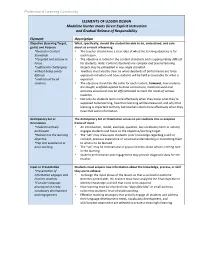
ELEMENTS of LESSON DESIGN Madeline Hunter Meets Direct Explicit Instruction and Gradual Release of Responsibility
Professional Learning Community ELEMENTS OF LESSON DESIGN Madeline Hunter meets Direct Explicit Instruction and Gradual Release of Responsibility Element Description Objective (Learning Target, What, specifically, should the student be able to do, understand, and care goals) and Purpose about as a result of learning. *Rooted in Content • The teacher should have a clear idea of what the learning objective is for Standards each lesson. *Targeted and narrow in • The objective is rooted in the content standards and is appropriately difficult Focus for students. Note: Content standards are complex and several learning *Sufficiently challenging targets may be embedded in any single standard. without being overly • Teachers must also be clear on what standards of performance are to be difficult expected and when and how students will be held accountable for what is *understood by all expected. students • The objective should be the same for each student; however, how students are taught, scaffolds applied to draw connections, materials used and activities structured may be differentiated to meet the needs of various students. • Not only do students learn more effectively when they know what they’re supposed to be learning, how their learning will be measured, and why that learning is important to them, but teachers teach more effectively when they have that same information. Anticipatory Set or The Anticipatory Set or Orientation serves to put students into a receptive Orientation frame of mind. *Students actively • An introduction, model, example, question, key vocabulary term or activity participate engages students and focus on the objective/learning target. *Relevant to the learning • The “set” may draw upon students’ prior knowledge regarding a skill or objective concept, previous experience or universal understanding in connecting them *Tap into experience or to what is to be learned. -

MADELINE Ludwig Bemelmans
MADELINE Ludwig Bemelmans A Study Guide Written by Garrett Christopher MADELINE TABLE OF CONTENTS For the Teacher. 1 Suggestions For Using Activities . 1 - 2 Synopsis . 3 Author / Illustrator Information . 3 Pre-Reading Activities . 4 Word Study . 5 Understanding the Story . 6 - 7 Understanding Pictures . 8 Story Frame . 9 Extension Activity. 10 - 13 Follow-Up Activities . 14 Suggestions For Further Reading . 15 Answer Key. 16 Notes . 17 Little Novel-Ties® are printed on recycled paper. The purchase of this study guide entitles an individual teacher to reproduce pages for use in a classroom. Reproduction for use in an entire school or school system or for commercial use is prohibited. Beyond the classroom use by an individual teacher, reproduction, transmittal or retrieval of this work is prohibited without written permission from the publisher. Copyright © 1992 by LEARNING LINKS MADELINE For the Teacher This reproducible Little Novel-Ties study guide consists of activities to use in conjunction with the book Madeline. The picture book and its corresponding guide can become an important element in your whole language reading program. The guide contains a synopsis; background information on the author and illustrator; suggested pre-reading activities; exercises that focus on vocabulary; visual literacy, story comprehension, and critical thinking skills; and extension activities that link the story to other curriculum areas. Suggestions For Using Activities Pre-Reading Activities – Before You Read These activities are designed to activate children’s prior knowledge and build concepts or background information. They will motivate children to read and help them to understand the story better. Children can discuss answers to the questions with a partner, in a small cooperative learning group or with the entire class. -

'The Nun Study'
Fall 2012 Volume 7 No. 3 ‘The Nun Study’ by Reverend Myles N. Sheehan, S.J., M.D. In the first years of the 20th century, Alois Alzheimer, a German neu- In This Issue rologist, cared for a middle-aged woman with a marked personality From the Offices 5 change, characterized by bizarre behavior and memory loss. This wom- of NRRO an died about five years after he first met her, years characterized by an Calendar 5 inexorable decline to a final stage in which she was bed-bound, re- Planning for the 6 quired total care and was unable to communicate meaningfully. After Future her death, Dr. Alzheimer studied the brain of his patient and described the changes in intellect, behavior and brain structure that characterize the disease now known by his name. Alzheimer’s disease is a particular type of dementia. Dementia, when “… God is pre- used as a diagnostic term in medicine, refers to the progressive loss of sent and active cognitive function in an individual. Thus dementing illnesses affect even in those memory, language, the ability to recognize and name individuals, sense of direction, personality and other aspects of what it means for us to who are con- think and reason. There are various kinds of dementing illnesses, but fused or unable to remember. Reverend Myles N. Sheehan, S.J., M.D. Hope and love Father Myles Sheehan, Sheehan will be S.J., M.D., (pictured right) hosting a five-part can remain is Provincial for the New miniseries on graceful England Province of the aging that can be alive even when Society of Jesus. -

Active Productions
visit us at Film and TV Production List www.ubcpactra.ca (Print in landscape format) Productions that qualify for discounts under the BC Indie Program are designated with “BC Indie” beside the Production name. Production Type Shooting Production Director Casting Director Background Stunt Union Manager Coordinator Representative 26 Hour Day Movie of the Week 5/3/2021 - Geoff Dodd David Winning TBD TBD CAYLEE FINCH 1950 Franklin Street 5/21/2021 Vancouver 604-257-4720 604-257-4739 A Christmas Treasure (2021) Movie of the Week 5/25/2021 - Michele Futerman Michael Robison TBD TBD Dianna Musil 1950 Franklin Street 6/11/2021 Vancouver 604-257-4720 604-257-4739 A Million Little Things - Season 3 TV Series - Episodic 8/27/2020 - Wayne Bennett Various Directors Clark & Page Lisa Ratke Trevor Jones MONIKA IWANEK 4200 North Fraser Way 5/17/2021 Burnaby 604-398-6655 604-398-6654 American Dreamer Feature Film 3/15/2021 - Christian Bruyere Paul Dektor Jackie Lind Jacqui Kaese Randy Lee ADRIENNE LAW 4700 Markham Street 4/21/2021 Victoria 778-406-0555 Batwoman - Season 2 TV Series - Episodic 9/3/2020 - Aren Ophoff Caroline Dries Candice Elzinga Doreen Ferreira Marshall Virtue MONIKA IWANEK 8035 Glenwood Brive - 3rd Floor 5/13/2021 Burnaby 604-647-5613 Big Sky, The - Season 1 TV Series - Episodic 8/27/2020 - Christina Toy Various Directors Coreen Mayrs Sandra Ken Dave Hospes DEBORAH RAY 803-19055 Airport Way 4/23/2021 Freeman Pitt Meadows 604-674-8196 Bonfire New Media 3/8/2021 - John Catron David Lowery Clark & Page Andrea Brown Lee Morrison CAYLEE FINCH 2880 Underhill Ave. -

The Flagellum and Flagellar Pocket of Trypanosomatids
Molecular & Biochemical Parasitology 115 (2001) 1–17 www.parasitology-online.com. Reviews: Parasite cell Biology: 1 The flagellum and flagellar pocket of trypanosomatids Scott M. Landfear *, Marina Ignatushchenko Department of Molecular Microbiology and Immunology, Oregon Health Sciences Uni6ersity, Portland, OR 97201, USA Received 9 November 2000; received in revised form 26 January 2001; accepted 5 March 2001 Abstract The flagellum and flagellar pocket are distinctive organelles present among all of the trypanosomatid protozoa. Currently, recognized functions for these organelles include generation of motility for the flagellum and dedicated secretory and endocytic activities for the flagellar pocket. The flagellar and flagellar pocket membranes have long been recognized as morphologically separate domains that are component parts of the plasma membrane that surrounds the entire cell. The structural and functional specialization of these two membranes has now been underscored by the identification of multiple proteins that are targeted selectively to each of these domains, and non-membrane proteins have also been identified that are targeted to the internal lumina of these organelles. Investigations on the functions of these organelle-specific proteins should continue to shed light on the unique biological activities of the flagellum and flagellar pocket. In addition, work has begun on identifying signals or modifications of these proteins that direct their targeting to the correct subcellular location. Future endeavors should further refine our knowledge of targeting signals and begin to dissect the molecular machinery involved in transporting and retaining each polypeptide at its designated cellular address. © 2001 Elsevier Science B.V. All rights reserved. Keywords: Trypanosomatid protozoa; Flagellum; Flagellar Pocket; Organelle-specific proteins; Review 1.