Experimental 2K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 the Volumetric Determination of Hydroxylamine
VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. I363 [CONTRIBUTION FROM THE CHEMICAL LABORATORYOF THE UNIVERSITY OF CALIFORNIA.1 THE VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. BY WILLIAMC. BRAY,MIBUM E. SIMPSONAND ANNA A. MACKENZIE. Received July 17, 1919 In the present investigation 3 volumetric methods of determining hydroxylamine in aqueous solution have been studied : The titanous salt method,' in which the hydroxylamine is reduced by excess titanous salt in acid solution with exclusion of air, and the excess titrated with permanganate. 2NH20H + Ti2(S04)3 = (NH4)2S04 + 4TiOS04 + HzS04. (I) The ferric salt method,2 in which the hydroxylamine is oxidized in an acid solution by excess of a ferric salt, the mixture is boiled and the fer- rous salt formed titrated with permanganate. 2NH20H + 2Fe@04)3 = N2O + 4FeS04 + 2H2S04 + H20. (2) The iodine method,3 in which the hydroxylamine is oxidized by iodine in a neutral solution, e. g., in the presence of disodium phosphate. 2NH20H + 212 = N2O + 4HI + H2O (3) or 2NH20H + 213- = N20 + 61- + 4H+ + HzO. Our first experiments, with the iodine method, yielded irregular results which could not be interpreted until the concentration of the hydroxyl- amine solution was accurately determined. An examination of the literature showed a rather unsatisfactory state of affairs. The advocates of the ferric sulfate method furnish evidence that it is perfectly reliable, but Leuba4 gives detailed experimental data to prove the contrary, and Adams5 states that he could not obtain reproducible results with it. The investigators who have used the iodine method consider it to be fairly satisfactory, but some of them state that it is not very accurate, and Rupp and Maeder6 have recently concluded that correct results are obtained only by a compensation of errors. -

A Fundamental Evaluation of the Atmospheric Pre-Leaching Section of the Nickel-Copper Matte Treatment Process
A FUNDAMENTAL EVALUATION OF THE ATMOSPHERIC PRE-LEACHING SECTION OF THE NICKEL-COPPER MATTE TREATMENT PROCESS by RODRICK MULENGA LAMYA Dissertation presented for the Degree of DOCTOR OF PHILOSOPHY (Extractive Metallurgical Engineering) in the Department of Process Engineering at the University of Stellenbosch, South Africa Promoter Prof. L. Lorenzen STELLENBOSCH March 2007 DECLARATION I the undersigned, hereby declare that the work contained in this dissertation is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. Signature: ............................................... Date: ....................................................... Copyright © 2007 Stellenbosch University All rights reserved i SYNOPSIS Nickel-Copper sulphide ores are the most important Platinum Group Metal bearing ores. The South African deposits are exceptionally rich in the platinum group metals (PGMs) and production of the PGMs is the primary purpose of treating these ores. The methods used in the recovery of the PGMs from the nickel-copper ores generally consists of ore concentration by physical techniques, pyrometallurgical concentration and hydrometallurgical extraction of the base metals followed by the PGMs. Pyrometallurgical concentration produces Ni-Cu matte, which is treated by hydrometallurgical processes to recover the nickel, copper, cobalt and the precious metals. In this study, the leaching behaviour of a Ni–Cu matte in CuSO4–H2SO4 solution during the repulping (pre-leach) stage at Impala Platinum Refineries was studied. The repulping stage is basically a non–oxidative atmospheric leach stage, in which nickel, iron and cobalt are partially dissolved, while the copper is precipitated. To understand the nature of the leaching process during this stage of the base metal refining operation, the effects of variations in the key process variables such as temperature, stirring rate, particle size, pulp density, residence time, initial copper and acid concentrations were investigated. -
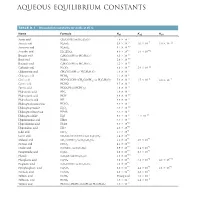
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Acids and Bases
Name Date Class CHAPTER 14 REVIEW Acids and Bases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Name the following compounds as acids: sulfuric acid a. H2SO4 sulfurous acid b. H2SO3 hydrosulfuric acid c. H2S perchloric acid d. HClO4 hydrocyanic acid e. hydrogen cyanide 2. H2S Which (if any) of the acids mentioned in item 1 are binary acids? 3. Write formulas for the following acids: HNO2 a. nitrous acid HBr b. hydrobromic acid H3PO4 c. phosphoric acid CH3COOH d. acetic acid HClO e. hypochlorous acid 4. Calcium selenate has the formula CaSeO4. H2SeO4 a. What is the formula for selenic acid? H2SeO3 b. What is the formula for selenous acid? 5. Use an activity series to identify two metals that will not generate hydrogen gas when treated with an acid. Choose from Cu, Ag, Au, Pt, Pd, or Hg. 6. Write balanced chemical equations for the following reactions of acids and bases: a. aluminum metal with dilute nitric acid ϩ → ϩ 2Al(s) 6HNO3(aq) 2Al(NO3)3(aq) 3H2(g) b. calcium hydroxide solution with acetic acid ϩ → ϩ Ca(OH)2(aq) 2CH3COOH(aq) Ca(CH3COO)2(aq) 2H2O(l ) MODERN CHEMISTRY ACIDS AND BASES 117 Copyright © by Holt, Rinehart and Winston. All rights reserved. Name Date Class SECTION 1 continued 7. Write net ionic equations that represent the following reactions: a. the ionization of HClO3 in water ϩ → ϩ ϩ Ϫ HClO3(aq) H2O(l ) H3O (aq) ClO3 (aq) b. NH3 functioning as an Arrhenius base ϩ → ϩ ϩ Ϫ NH3(aq) H2O(l ) ← NH4 (aq) OH (aq) 8. -

Process for the Preparation of Chacogens and Chalcogenide Alloys of Controlled Average Crystallite Size
Europaisches Patentamt European Patent Office @ Publication number: 0 1 60 493 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 85302825.6 © Int. CI.4: C 01 B 19/02 _ C 01 B 19/04 © Date of filing: 23.04.85 © Priority: 23.04.84 US 603019 © Applicant: XEROX CORPORATION Xerox Square - 020 Rochester New York 14644(US) @ Date of publication of application: 06.11.85 Bulletin 85/45 @ Inventor: Badesha, Santokh Singh 665 Cievenger Road @ Designated Contracting States: Ontario New York 1451 9(US) DE FR GB © Inventor: Fekete, George Thomas 111 Dale Road Rochester New York 14625IUS) © Representative: Goode, Ian Roy et al, European Patent Attorney do Rank Xerox Limited Patent Department Rank Xerox House 338 Euston Road London NW1 3BH(GB) (64) Process for the preparation of chacogens and chalcogenide alloys of controlled average crystallite size. A process for the preparation of chalcogens and chal- cogenide alloys which comprises subjecting the correspond- ing esters to a reduction or coreduction reaction, for example using hydrazine, at a temperature between about 40°C and 135°C. The morphology/crystallite size of the product is controlled by suitably selecting a temperature within this range, depending on the particular chalcogen or alloy being prepared. The product is either a non-crystalline material, or has an average crystallite size between about 15 and 52 nm. This invention relates to a process for the preparation of chalcogens and chalcogenide alloys, the process being of the kind which comprises subjecting the corresponding esters to a reduction or coreduction reaction. Such a process for preparing high purity tellurium is described in EP-A-0 102 753, and such a process for preparing high purity chalcogenide alloys, such as selenium/tellurium alloys, is described in EP-A-0 101 238. -

1,2-Cyclohexanedione Dioxime
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Why Nature Chose Selenium Hans J
Reviews pubs.acs.org/acschemicalbiology Why Nature Chose Selenium Hans J. Reich*, ‡ and Robert J. Hondal*,† † University of Vermont, Department of Biochemistry, 89 Beaumont Ave, Given Laboratory, Room B413, Burlington, Vermont 05405, United States ‡ University of WisconsinMadison, Department of Chemistry, 1101 University Avenue, Madison, Wisconsin 53706, United States ABSTRACT: The authors were asked by the Editors of ACS Chemical Biology to write an article titled “Why Nature Chose Selenium” for the occasion of the upcoming bicentennial of the discovery of selenium by the Swedish chemist Jöns Jacob Berzelius in 1817 and styled after the famous work of Frank Westheimer on the biological chemistry of phosphate [Westheimer, F. H. (1987) Why Nature Chose Phosphates, Science 235, 1173−1178]. This work gives a history of the important discoveries of the biological processes that selenium participates in, and a point-by-point comparison of the chemistry of selenium with the atom it replaces in biology, sulfur. This analysis shows that redox chemistry is the largest chemical difference between the two chalcogens. This difference is very large for both one-electron and two-electron redox reactions. Much of this difference is due to the inability of selenium to form π bonds of all types. The outer valence electrons of selenium are also more loosely held than those of sulfur. As a result, selenium is a better nucleophile and will react with reactive oxygen species faster than sulfur, but the resulting lack of π-bond character in the Se−O bond means that the Se-oxide can be much more readily reduced in comparison to S-oxides. -

H2seo3. Arxiv:1901.03765V1
Crystal Structure Elucidation of the Novel Molecular-Inorganic Polymer K2SeO4 · H2SeO3. Oscar S. Hernández-Daguer 1,2, Charles L. Barnes3, Samuel P. Hernández-Rivera4, and Jorge L. Ríos-Steiner 5 1Department of Physics, University of Massachusetts, Amherst, Massachusetts 01003, United States, e-mail: [email protected], [email protected] 2Department of Physics, University of Puerto Rico, Mayagüez, Puerto Rico 00681-9000, United States, e-mail: [email protected], [email protected], phone: +1 787 228 1380. 3Department of Chemistry, University of Missouri, Columbia, Missouri 65211, United States. 4ALERT-II DHS Center of Excellence for Explosives Research, Department of Chemistry, University of Puerto Rico, Mayagüez, Puerto Rico, 00681-9000, United States. 5Laboratory of Crystallography and Synthesis of New Materials, Department of Chemistry, University of Puerto Rico, Mayagüez, Puerto Rico, 00681-9000 United States, e-mail: [email protected], phone: +1 787 832 4040 ext 2538, Fax: +1 787 265 3849. January 15, 2019 Abstract The orthorhombic crystal structure of the novel molecular-inorganic polymer K2SeO4 · H2SeO3 was elucidated using single-crystal X-ray diffraction with MoKα radiation (λ= 0.71073 Å), performed at 100 and 298.15 K. The reported data is at 100 K since there were no struc- tural differences as compared to the room temperature. The technique revealed K2SeO4 ·H2SeO3 crystals to have space group P bcm with unit cell dimensions a = 8.8672(17) Å, b = 7.3355(14) Å, c = 11.999(2) Å and Z = 4. The unit cell volume obtained was V = 780.5(3) Å3 3 arXiv:1901.03765v1 [cond-mat.mtrl-sci] 11 Jan 2019 with a calculated density Dc = 2:980Mg=m . -

WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61P 3/04 (2006.01) A61K 33/40 (2006.01) kind of national protection available): AE, AG, AL, AM, A61P 9/10 (2006.01) A61K 38/44 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 35/74 (2015.01) A61K 31/17 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/US20 16/034973 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 3 1 May 2016 (3 1.05.2016) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/169,480 1 June 2015 (01 .06.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/327,283 25 April 2016 (25.04.2016) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: XENO BIOSCIENCES INC. -

Preparation of Monodisperse Se Colloid Spheres and Se Nanowires
Nano Res (2008) 1: 403 411 DOI 10.1007/s12274-008-8040-5 00403 Research Article Preparation of Monodisperse Se Colloid Spheres and Se Nanowires Using Na2SeSO3 as Precursor Liping Liu, Qing Peng, and Yadong Li( ) Department of Chemistry, Tsinghua University, Beijing 100084, China Received: 3 September 2008 / Revised: 19 September 2008 /Accepted: 19 September 2008 ©Tsinghua Press and Springer-Verlag 2008. This article is published with open access at Springerlink.com ABSTRACT Nearly monodisperse spherical amorphous Se colloids are prepared by the dismutation of Na2SeSO3 solution at room temperature; by altering the pH of the solution, amorphous Se colloid spheres with sizes of about 120 nm, 200 nm, 300 nm, and 1 μm can be obtained. Se@Ag2Se core/shell spheres are successfully synthesized by using the obtained amorphous Se (a-Se) spheres as templates, indicating the potential applications of these Se nanomaterials in serving as soft templates for other selenides. Meanwhile, selenium nanowires are obtained through a “solid-solution-solid” growth process by dispersing the prepared Se spheres in ethanol. This simple and environmentally benign approach may offer more opportunities in the synthesis and applications of nanocrystal materials. KEYWORDS Na2SeSO3, dismutation, amorphous Se (a-Se) spheres, trigonal Se (t-Se) nanowires Introduction structures, as it is easily removed [4, 5]. Furthermore, selenium is a relatively active material and can react Materials which are composed of the element selenium with a variety of chemical reagents to form other play important roles in the fields of photonics materials, such as Ag Se, and CdSe [1, 6, 7], which and electronics due to its special photoconductive 2 possess wide potential applications as soft templates properties [1, 2]; the electrical conductivity of selenium for functional selenides. -

Risk-Based Closure Guide Draft
Risk-based Closure Guide July 1, 2021 Office of Land Quality Indiana Department of Environmental Management Disclaimer This Nonrule Policy Document (NPD) is being established by the Indiana Department of Environmental Management (IDEM) consistent with its authority under IC 13-14-1-11.5. It is intended solely as guidance and shall be used in conjunction with applicable rules or laws. It does not replace applicable rules or laws, and if it conflicts with these rules or laws, the rules or laws shall control. Pursuant to IC 13-14-1-11.5, this NPD will be available for public inspection for at least forty-five (45) days prior to presentation to the appropriate State Environmental Board, and may be put into effect by IDEM thirty (30) days afterward. If the NPD is presented to more than one board, it will be effective thirty (30) days after presentation to the last State Environmental Board. IDEM also will submit the NPD to the Indiana Register for publication. 2 Table of Contents 1 Introduction ..................................................................................................................................... 7 1.1 Applicability .............................................................................................................................. 8 1.2 Types of Closure ...................................................................................................................... 9 1.3 Process Overview ...................................................................................................................