AP Environmental Science Air Pollution Formulas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 the Volumetric Determination of Hydroxylamine
VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. I363 [CONTRIBUTION FROM THE CHEMICAL LABORATORYOF THE UNIVERSITY OF CALIFORNIA.1 THE VOLUMETRIC DETERMINATION OF HYDROXYLAMINE. BY WILLIAMC. BRAY,MIBUM E. SIMPSONAND ANNA A. MACKENZIE. Received July 17, 1919 In the present investigation 3 volumetric methods of determining hydroxylamine in aqueous solution have been studied : The titanous salt method,' in which the hydroxylamine is reduced by excess titanous salt in acid solution with exclusion of air, and the excess titrated with permanganate. 2NH20H + Ti2(S04)3 = (NH4)2S04 + 4TiOS04 + HzS04. (I) The ferric salt method,2 in which the hydroxylamine is oxidized in an acid solution by excess of a ferric salt, the mixture is boiled and the fer- rous salt formed titrated with permanganate. 2NH20H + 2Fe@04)3 = N2O + 4FeS04 + 2H2S04 + H20. (2) The iodine method,3 in which the hydroxylamine is oxidized by iodine in a neutral solution, e. g., in the presence of disodium phosphate. 2NH20H + 212 = N2O + 4HI + H2O (3) or 2NH20H + 213- = N20 + 61- + 4H+ + HzO. Our first experiments, with the iodine method, yielded irregular results which could not be interpreted until the concentration of the hydroxyl- amine solution was accurately determined. An examination of the literature showed a rather unsatisfactory state of affairs. The advocates of the ferric sulfate method furnish evidence that it is perfectly reliable, but Leuba4 gives detailed experimental data to prove the contrary, and Adams5 states that he could not obtain reproducible results with it. The investigators who have used the iodine method consider it to be fairly satisfactory, but some of them state that it is not very accurate, and Rupp and Maeder6 have recently concluded that correct results are obtained only by a compensation of errors. -

A Fundamental Evaluation of the Atmospheric Pre-Leaching Section of the Nickel-Copper Matte Treatment Process
A FUNDAMENTAL EVALUATION OF THE ATMOSPHERIC PRE-LEACHING SECTION OF THE NICKEL-COPPER MATTE TREATMENT PROCESS by RODRICK MULENGA LAMYA Dissertation presented for the Degree of DOCTOR OF PHILOSOPHY (Extractive Metallurgical Engineering) in the Department of Process Engineering at the University of Stellenbosch, South Africa Promoter Prof. L. Lorenzen STELLENBOSCH March 2007 DECLARATION I the undersigned, hereby declare that the work contained in this dissertation is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. Signature: ............................................... Date: ....................................................... Copyright © 2007 Stellenbosch University All rights reserved i SYNOPSIS Nickel-Copper sulphide ores are the most important Platinum Group Metal bearing ores. The South African deposits are exceptionally rich in the platinum group metals (PGMs) and production of the PGMs is the primary purpose of treating these ores. The methods used in the recovery of the PGMs from the nickel-copper ores generally consists of ore concentration by physical techniques, pyrometallurgical concentration and hydrometallurgical extraction of the base metals followed by the PGMs. Pyrometallurgical concentration produces Ni-Cu matte, which is treated by hydrometallurgical processes to recover the nickel, copper, cobalt and the precious metals. In this study, the leaching behaviour of a Ni–Cu matte in CuSO4–H2SO4 solution during the repulping (pre-leach) stage at Impala Platinum Refineries was studied. The repulping stage is basically a non–oxidative atmospheric leach stage, in which nickel, iron and cobalt are partially dissolved, while the copper is precipitated. To understand the nature of the leaching process during this stage of the base metal refining operation, the effects of variations in the key process variables such as temperature, stirring rate, particle size, pulp density, residence time, initial copper and acid concentrations were investigated. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Why Nature Chose Selenium Hans J
Reviews pubs.acs.org/acschemicalbiology Why Nature Chose Selenium Hans J. Reich*, ‡ and Robert J. Hondal*,† † University of Vermont, Department of Biochemistry, 89 Beaumont Ave, Given Laboratory, Room B413, Burlington, Vermont 05405, United States ‡ University of WisconsinMadison, Department of Chemistry, 1101 University Avenue, Madison, Wisconsin 53706, United States ABSTRACT: The authors were asked by the Editors of ACS Chemical Biology to write an article titled “Why Nature Chose Selenium” for the occasion of the upcoming bicentennial of the discovery of selenium by the Swedish chemist Jöns Jacob Berzelius in 1817 and styled after the famous work of Frank Westheimer on the biological chemistry of phosphate [Westheimer, F. H. (1987) Why Nature Chose Phosphates, Science 235, 1173−1178]. This work gives a history of the important discoveries of the biological processes that selenium participates in, and a point-by-point comparison of the chemistry of selenium with the atom it replaces in biology, sulfur. This analysis shows that redox chemistry is the largest chemical difference between the two chalcogens. This difference is very large for both one-electron and two-electron redox reactions. Much of this difference is due to the inability of selenium to form π bonds of all types. The outer valence electrons of selenium are also more loosely held than those of sulfur. As a result, selenium is a better nucleophile and will react with reactive oxygen species faster than sulfur, but the resulting lack of π-bond character in the Se−O bond means that the Se-oxide can be much more readily reduced in comparison to S-oxides. -

Risk-Based Closure Guide Draft
Risk-based Closure Guide July 1, 2021 Office of Land Quality Indiana Department of Environmental Management Disclaimer This Nonrule Policy Document (NPD) is being established by the Indiana Department of Environmental Management (IDEM) consistent with its authority under IC 13-14-1-11.5. It is intended solely as guidance and shall be used in conjunction with applicable rules or laws. It does not replace applicable rules or laws, and if it conflicts with these rules or laws, the rules or laws shall control. Pursuant to IC 13-14-1-11.5, this NPD will be available for public inspection for at least forty-five (45) days prior to presentation to the appropriate State Environmental Board, and may be put into effect by IDEM thirty (30) days afterward. If the NPD is presented to more than one board, it will be effective thirty (30) days after presentation to the last State Environmental Board. IDEM also will submit the NPD to the Indiana Register for publication. 2 Table of Contents 1 Introduction ..................................................................................................................................... 7 1.1 Applicability .............................................................................................................................. 8 1.2 Types of Closure ...................................................................................................................... 9 1.3 Process Overview ................................................................................................................... -

Sulfur Dioxide and “Sulfurous Acid” Solutions. S + O 2 → SO2 (G
Sulfur Dioxide and “Sulfurous Acid” Solutions. S + O2 → SO2 (g) Structure: sp2 at sulfur, two used for σ bonds, one for lone pair. : δ+ S - δ- δ O O Liquid SO2: Easy to get (bp = -10 °C) and easy to keep liquid at room T under slight pressure. It is quite a good solvent; SO2 is a polar molecule, and the liquid is associated by dipole-dipole attraction. Dissolves polar molecules and ionic compounds, and easy to remove by evaporation. Aqueous solutions: SO2 is soluble in water. Most of it stays in the form of hydrogen bonded “hydrate” (see CO2). At equilibrium in neutral water (no added base) a small portion of it reacts: - H+ - H+ - 2- SO2 (aq) + H2O HO-SO2 SO3 (sulfite - pyramidal) + H+ + H+ - H-SO3 (bisulfite) - 2- 2- Also, 2 HSO3 S2O5 + H2O (structure = [O2S-SO3] ) Many salts of all these anions are known. Complexes formed with sulfite ion and SO2 itself. Sulfur Trioxide and Sulfuric Acid. 2 SO2 + O2 → 2 SO3 (g) Condenses to a liquid at room T, liquid contains monomer and trimer. O O S O O O O O S S S O O O O O Also get solid polymers. SO3 is a powerful oxidant, with water, gives sulfuric acid – H2SO4. A viscous, hydrogen bonded liquid; which is a good strongly acidic solvent. In dilute solutions in water it is a dibasic acid. - H+ - H+ - 2- H2SO4 (aq) HSO4 (bisulfate) SO4 (sulfate) + H+ + H+ Many salts known for both anions. Sulfate can be monodentate or bidentate ligand. SO3 dissolves in concentrated H2SO4 to give “fuming” sulfuric acid which contains some pyrosulfuric acid – VERY CORROSIVE H2SO4 + SO3 H2S2O7 Halides of Sulfur Sulfur hexafluoride (SF6) is a gas a stp. -

Atmospheric Chemistry Lecture 2
AtmosphericAtmospheric ChemistryChemistry LectureLecture 2:2: TroposphericTropospheric ChemistryChemistry andand AerosolsAerosols Jim Smith Atmospheric Chemistry Division / NCAR [email protected] Outline for Second Lecture Tropospheric Chemistry and Aerosols Primary and secondary pollutants Sulfur chemistry Nitrogen chemistry Acid rain Chemistry of the background troposphere: • CO • Methane • Formaldehyde Urban Air Pollution • Hydrocarbon oxidation • Ozone Isopleth plots • Smog meteorology Tropospheric Chemistry Primary and Secondary Pollutants and their impacts Primary pollutants are those emitted directly from sources • CO: affects oxygen-carrying capacity of blood (but not at urban concentrations) • NO: secondary reactions • NO 2: 5% in urban, much higher for biomass burning • SO 2: respiratory constriction (asthma, bronchitis) • NH 3: aerosol formation • soot: visibility reduction, PAH is carcinogen • isoprene: emitted from plants, leads to ozone and makes a certain President think the ozone problem is caused by trees. • vanillan: yummy smell from Ponderosa Pine Secondary pollutants are those formed in the atmosphere by chemical interactions among primary pollutants and normal atmospheric constituents. • NO 2 (95%): lung damage •H2SO 4: Aerosol formation, visibility reduction and acid rain • PAN: hurts your eyes, damage to plants •O3: damage to plants & plastics, lung damage (only short-term?) Tropospheric Chemistry Sulfur Cycle Image courtesy of Hugh Powell, Univ. of Durham, UK Tropospheric Chemistry Sulfur Cycle SO 2 can -

ABSTRACT RUDD, HAYDEN. Assessing the Vulnerability Of
ABSTRACT RUDD, HAYDEN. Assessing the Vulnerability of Coastal Plain Groundwater to Flood Water Intrusion using High Resolution Mass Spectrometry. (Under the direction of Dr. Elizabeth Guthrie Nichols). Communities in the North Carolina Coastal Plain (NCCP) depend on safe and reliable groundwater for private well use, agriculture, industry, and livelihoods. Although storm intensity and frequency are predicted to increase in coastal areas, the risk of surficial and confined aquifer contamination from extreme storms is not understood. In September 2018, Hurricane Florence caused extensive flooding across the NCCP for several weeks. The North Carolina Department of Environmental Quality (NCDEQ) Groundwater Management Branch had just completed sampling of some wells in their monitoring network when Hurricane Florence made landfall. NCDEQ returned to these wells, particularly those flooded by Hurricane Florence, for post-flood sampling. These groundwater samples were analyzed by NCDEQ for regulated semi-volatile organics with few to any detections of regulated organic contaminants. NCDEQ provided NC State the same sample extracts for analysis by high resolution mass spectrometry (HRMS). This research reports on the non-targeted and suspect-screening HRMS analyses of groundwater from nested monitoring wells. Some monitoring well sites experienced flooding during the study period, and some did not. The goal of this research was to advance our understanding of coastal aquifer susceptibility to flooding by producing the first comprehensive organic chemical profiles of coastal aquifers and by determining if aquifers have distinct organic chemical profiles that change after flooding events. This study used HRMS analyses to produce the first comprehensive organic chemical profiles of 11 aquifers in the coastal plain. -

The Chemical and Preservative Properties of Sulfur Dioxide Solution for Brining Fruit
The Chemical and Preservative Properties of Sulfur Dioxide Solution for Brining Fruit Circular of Information 629 June 1969 Agricultural Experiment Station, Oregon State University, Corvallis The Chemical and Preservative Properties of Sulfur Dioxide Solution for Brining Fruit C. H. PAYNE, D. V. BEAVERS, and R. F. CAIN Department of Food Science and Technology Sweet cherries and other fruits are preserved in sul- control are given in papers by Waiters et al. (1963) and fur dioxide solutions for manufacture into maraschino, Yang et al. (1966). cocktail, and glace fruit. The sulfur dioxide solution, Sulfur dioxide and calcium are directly related to commonly called brine, may be prepared from liquid brined cherry quality. Improper use of these chemicals sulfur dioxide, sodium bisulfite, or sodium metabisulfite may result in cherries that are soft, poorly bleached, or using alkali or acid to control pH. Calcium salts such as spoiled due to fermentation. It is the purpose of this calcium hydroxide, calcium carbonate, and calcium chlo- circular to show how the basic chemical and preservative ride are added to the brine to prevent cracking and pro- components of brine solutions are afifected during prep- mote firming of the fruit tissue through interaction with aration, storage, and use. pectic materials. Directions for brine preparation and Chemical Properties of Sulfur Dioxide Solutions When sulfur dioxide or materials containing sulfur range of sulfur dioxide concentrations, the relative dis- dioxide (bisulfite or metabisulfite) are dissolved in tribution of the sulfur dioxide ionic forms is constant. water, three types of chemical substances are formed: Within the initial pH range used for brining cher- sulfurous acid (H2SO3),1 bisulfite (HSO3"), and sulfite ries, there is a predominance of calcium bisulfite (SO~). -

Nickel and Its Alloys
National Bureau of Standards Library, E-01 Admin. Bldg. IHW 9 1 50CO NBS MONOGRAPH 106 Nickel and Its Alloys U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics—Laboratory Astrophysics^—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -
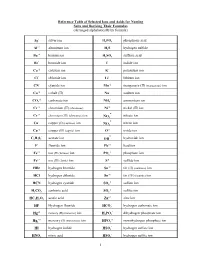
1 Reference Table of Selected Ions and Acids for Naming Salts and Deriving Their Formulas
Reference Table of Selected Ions and Acids for Naming Salts and Deriving Their Formulas (Arranged alphabetically by formula) + Ag silver ion HP34O phosphoric acid +3 Al aluminum ion HS2 hydrogen sulfide +2 Ba barium ion HS24O sulfuric acid Br- bromide ion I- iodide ion Ca+2 calcium ion K+ potassium ion Cl- chloride ion Li+ lithium ion CN- cyanide ion Mn+2 manganese (II) (manganous) ion Co+2 cobalt (II) Na+ sodium ion -2 + CO3 carbonate ion NH4 ammonium ion Cr+2 chromium (II) (chromous) Ni+2 nickel (II) ion - Cr+3 chromium (III) (chromous) ion nitrate ion NO3 - Cu+ copper (I) (cuprous) ion nitrite ion NO2 Cu+2 copper (II) (cupric) ion O-2 oxide ion - - C23H02 acetate ion OH hydroxide ion F- fluoride ion Pb+2 lead ion +2 -3 Fe iron (II) (ferrous) ion PO4 phosphate ion Fe+3 iron (III) (ferric) ion S-2 sulfide ion HBr hydrogen bromide Sn+2 tin (II) (stannous) ion HCl hydrogen chloride Sn+4 tin (IV) (stannic) ion -2 HCN hydrogen cyanide SO4 sulfate ion -2 HC23O carbonic acid SO3 sulfite ion +2 HC232HO acetic acid Zn zinc ion - HF Hydrogen fluoride HCO3 hydrogen carbonate ion +2 - Hg mercury (II) (mercuric) ion HP24O dihydrogen phosphate ion +2 -2 Hg2 mercury (I) (mercurous) ion HPO4 monohydrogen phosphate ion - HI hydrogen iodide HSO4 hydrogen sulfate ion - HNO3 nitric acid HSO3 hydrogen sulfite ion 1 NAMES AND FORMULAS FOR SOME COMMON ACIDS AND ANIONS (BASES) DERIVED FROM THEM HBr Hydrobromic acid Br- bromide ion (Hydrogen bromide) HCl Hydrochloric acid Cl- chloride ion (Hydrogen chloride) HCN Hydrocyanic acid CN - cyanide ion (Hydrogen cyanide) -

Class 562 Organic Compounds -- Part of the Class 532-570 Series 562 - 1
CLASS 562 ORGANIC COMPOUNDS -- PART OF THE CLASS 532-570 SERIES 562 - 1 562 ORGANIC COMPOUNDS -- PART OF THE CLASS 532-570 SERIES MOC NOTES 8 .Phosphorus acids or salts This Class 562 is... This Class 562 is considered to be an thereof (i.e., compounds integral part of Class 260 (see the Class having -XH, wherein X is 260 schedule for the position of this chalcogen, attached directly Class in schedule hierarchy). This Class to phosphorus by nonionic retains all pertinent definitions and bonding and wherein the class lines of Class 260. hydrogen may be replaced by a This Class 562 is... substituted or unsubstituted ammonium or by a group IA or IIA light metal) 9 ..Sulfur attached directly to the ORGANIC COMPOUNDS (CLASS 532, phosphorus by nonionic bonding SUBCLASS 1) 10 ..Nitrogen attached directly to 1 .Persulphonic acids or salts the phosphorus by nonionic thereof (i.e., compounds bonding having the -S(=O)(=O) O-OH group, wherein the hydrogen 11 ..Nitrogen attached indirectly to may be replaced by a group IA the phosphorus by nonionic or IIA light metal, or by bonding substituted or unsubstituted 12 ...Plural phosphori attached ammonium) indirectly to each other by 2 .Percarboxylic acids or salts nonionic bonding thereof (i.e., compounds 13 ....Plural phosphori bonded having the -C(=O)-O OH group, directly to the same carbon wherein the hydrogen may be 14 ....Additional nitrogen attached replaced by a group IA or IIA indirectly to the phosphorus light metal, or by substituted by nonionic bonding or unsubstituted ammonium) 15 ...The nitrogen