Johnson & Johnson 2005 Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Benadryl Allergy Relief Plus Decongestant Capsules
Distance between cutted line/middel of the spot = 51.6 mm spot = 12 x 2 centered in the margin of 14 mm le f 14 mm GB Benadryl Allergy Relief Plus Decon Caps 12s PIL MAH Update CRD 6738 t margin 23.06.2021 English 402428175_r0 N/A 1 Brochure/Leaet/Insert 145 x 250 mm Capsules are a medicine which are used to blood pressure). 364350C IM_Val-de-Reuil_Upper Normandy_France relieve the symptoms of hay fever and similar ■ If you have glaucoma (increased pressure allergic conditions. The capsules contain in the eye). CTE LITE 364350B Westrock Saint Pierre des Corps pseudoephedrine hydrochloride, which is a ■ If you have an overactive thyroid gland. 845 Oset Printing decongestant that relieves nasal and sinus ■ If you have severe kidney problems. 7996903 PAPER congestion and acrivastine which is an ■ If you are taking, or have taken in the last EMEA_2021_00024796_003 PC-0002778 antihistamine that helps relieve allergy two weeks, drugs for depression known symptoms such as sneezing, runny nose and as Monoamine Oxidase Inhibitors CAPSULES watery eyes. (MAOIs) or Reverse Inhibitors of Acrivastine 8mg & Pseudoephedrine 60mg Monoamine Oxidase 2 Before taking this (RIMAs). ■ This medicine is used to relieve the symptoms ■ If you are taking any cough or of hay fever and similar allergic conditions. cold medicines. PANTONE PANTONE medicine 356 C 287 C ■ This medicine is for use by adults and If any of these apply to you, get advice from children aged 12 - 65 years. This medicine is suitable for most adults under LITHO OFFSET LITHO OFFSET 65 years old and children aged 12 years and a doctor or pharmacist without taking ■ Do not take this medicine: REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

Learner Notification International Society for Heart & Lung
Learner Notification International Society for Heart & Lung Transplantation (ISHLT) 41st Annual Meeting & Scientific Sessions Virtual Experience April 24 – 28, 2021 Live Virtual Acknowledgement of Financial Commercial Support Abbott Medtronic United Therapeutics Acknowledgement of In-Kind Commercial Support No in-kind commercial support was received for this educational activity. Satisfactory Completion Learners must complete an evaluation form to receive a certificate of completion. Your chosen sessions must be attended in their entirety as partial credit of individual sessions is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement. Physicians (ACCME) The International Society for Heart and Lung Transplantation (ISHLT) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Credit Designation Statement - ISHLT designates this live virtual activity for a maximum of 32.00 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Accreditation Statement In support of improving patient care, this activity has been planned and implemented by Amedco LLC and ISHLT. Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. Nurses (ANCC) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 ANCC contact hours. Pharmacists (ACPE) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 knowledge-based CPE contact hours. -

2019 Health for Humanity Report
2019 Health for Humanity Report Progress in Sustainability Report Summary Contents Message from Our Chairman and CEO 3 Sustainability Approach 4 2019 Year in Brief 5 Better Health for All: Tackling the World’s 6 Toughest Health Challenges Better Health for All: Access, Community 7 Health & Innovation Responsible Business Practices 8 Environmental Health 9 UNICEF, the Government of Vietnam and Johnson & Johnson are partnering on a national program to train more than 500 ethnic minority midwives in remote regions to provide effective maternal and child health interventions including early essential newborn care in village clinics and homes. Photo by Paul Bettings Front cover Volunteers, frontline health workers and government officials at the launch of the Umurinzi vaccination program in Rwanda. In October 2019, Johnson & Johnson committed to donating up to 700,000 regimens of Janssen’s investigational Ebola vaccine to support the Ebola outbreak response in Rwanda and the Democratic Republic of the Congo. Photos by Rwanda Ministry of Health 2019 Health2017 for Health Humanity for HumanityReport Summary Report 33 Message from Our Chairman and CEO Dear Johnson & Johnson Stakeholders, We know this mission will always be unfinished, and affirmed unequivocally that there is a fundamental that we will occasionally fall short. But that only serves connection between serving all stakeholders and 2019 was a year of profound change and great contrasts as motivation to move faster than we’ve ever moved generating sustainable, long-term value. around the globe. before in making bigger strides toward some of our most ambitious goals. And as we’ve detailed in this Report, we The demands for global healthcare and responsible We saw unprecedented innovation and encouraging have plenty of positive momentum worth recognizing. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

2Nd Quarter 2015 Earnings Call Presentation
2nd Quarter 2015 Earnings Call Presentation July 14, 2015 1 Louise Mehrotra Vice President Investor Relations 2 Note on Forward-Looking Statements These presentations contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, future operating and financial performance, product development, market position and business strategy. The viewer is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to, economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges and uncertainties inherent in new product development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; the ability of the company to successfully execute strategic plans; impact of business combinations and divestitures; challenges to patents; the impact of patent expirations; significant adverse litigation or government action, including related to product liability claims; changes to applicable laws and regulations, including global health care reforms; trends toward health -

No. 33981 2 No
Pretoria, 4 February 2011 Februarle No. 33981 2 No. 33981 GOVERNMENT GAZETTE, 4 FEBRUARY 2011 IMPORTANT NOTICE The Government Printing Works will not be held responsible for faxed documents not received due to errors on the fax machine or faxes received which are unclear or incomplete. Please be advised that an "OK" slip, received from a fax machine, will not be accepted as proof that documents were received by the GPW for printing. If documents are faxed to the GPW it will be the sender's respon sibility to phone and confirm that the documents were received in good order. Furthermore the Government Printing Works will also not be held responsible for cancellations and amendments which have not been done on original documents received from clients. CONTENTS INHOUD Bladsy Koerant Page Gazette No. No. No. No. No. No. GENERAL NOTICE ALGEMENEKENNISGEWING Health, Department of Gesondheld, Departement van General Notice A/gemene Kennisgewing 58 Medicines and Related Substances Act 58 Wet op Beheer van Medisyne en (101/1965): Medicines Control Council: Verwante Stowwe (101/1965): Conditions of registration of a medicine Medisynebeheerraad: Voorwaardes vir in terms of the provisions of section die registrasie van 'n medisyne in terme 15 (7) ..................................................... .. 3 33981 van die bepalings van artikel 15 (7) ........ 4 33981 STAATSKOERANT, 4 FEBRUARIE 2011 No. 33981 3 GENERAL NOTICE ALGEMENE KENNISGEWING NOTICE 58 OF 2011 MEDICINES CONTROL COUNCIL CONDITIONS OF REGISTRATION OF A MEDICINE IN TERMS OF THE PROVISIONS OF SECTION 15(7) OF THE MEDICINES AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965) 1. The applicant shall ensure that the medicine is manufactured and controlled in terms of the current Good Manufacturing Practices as determined by Council 2. -

Gender, the Status of Women, and Family Structure in Malaysia
Malaysian Journal of EconomicGender, Studies the Status 53(1): of Women,33 - 50, 2016 and Family Structure in Malaysia ISSN 1511-4554 Gender, the Status of Women, and Family Structure in Malaysia Charles Hirschman* University of Washington, Seattle Abstract: This paper addresses the question of whether the relatively high status of women in pre-colonial South-east Asia is still evident among Malay women in twentieth century Peninsular Malaysia. Compared to patterns in East and South Asia, Malay family structure does not follow the typical patriarchal patterns of patrilineal descent, patrilocal residence of newly married couples, and preference for male children. Empirical research, including ethnographic studies of gender roles in rural villages and demographic surveys, shows that women were often economically active in agricultural production and trade, and that men occasionally participated in domestic roles. These findings do not mean a complete absence of patriarchy, but there is evidence of continuity of some aspects of the historical pattern of relative gender equality. The future of gender equality in Malaysia may depend as much on understanding its past as well as drawing lessons from abroad. Keywords: Family, gender, marriage, patriarchy, women JEL classification: I3, J12, J16, N35 1. Introduction In the introduction to her book onWomen, Politics, and Change, Lenore Manderson (1980) said that the inspiration for her study was the comment by a British journalist that the participation of Malay women in rallies, demonstrations, and the nationalist movement during the late 1940s was the most remarkable feature of post-World War II Malayan politics. The British journalist described the role of Malay women in the nationalist movement as “challenging, dominant, and vehement in their emergence from meek, quiet roles in the kampongs, rice fields, the kitchens, and nurseries” (Miller, 1982, p. -

IN the UNITED STATES DISTRICT COURT for the DISTRICT of DELAWARE ALZA CORPORATION and JANSSEN PHARMACEUTICALS, INC., Plaintiffs
Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 1 of 20 PageID #: 24 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ALZA CORPORATION and ) JANSSEN PHARMACEUTICALS, INC., ) ) Plaintiffs, ) ) v. ) Civil Action No. ____________ ) REDACTED PUBLIC VERSION AMNEAL PHARMACEUTICALS OF ) NEW YORK, LLC and ) AMNEAL PHARMACEUTICALS LLC, ) ) Defendants. ) COMPLAINT In this patent infringement action, Plaintiffs ALZA Corporation ("ALZA") and Janssen Pharmaceuticals, Inc. (collectively "Plaintiffs"), for their complaint against Defendants Amneal Pharmaceuticals of New York, LLC ("Amneal Pharms. NY") and Amneal Pharmaceuticals LLC ("Amneal Pharms. LLC") (collectively, "Amneal"), allege as follows: NATURE OF THE ACTION 1. This is an action for patent infringement under the patent laws of the United States, Title 35, United States Code, in response to, inter alia, the submission by Amneal of Abbreviated New Drug Application ("ANDA") No. 207515, with the U.S. Food and Drug Administration ("FDA") seeking approval to manufacture and sell a generic version of CONCERTA® prior to the expiration of U.S. Patent No. 8,163,798 ("the '798 patent") and U.S. Patent No. 9,144,549 ("the '549 patent"). PARTIES 2. Plaintiff ALZA is a Delaware corporation, having its principal place of business at 700 Eubanks Drive, Vacaville, California 95688. Case 1:16-cv-00914-UNA Document 3 Filed 10/07/16 Page 2 of 20 PageID #: 25 REDACTED PUBLIC VERSION 3. Plaintiff Janssen Pharmaceuticals, Inc. ("Janssen") is a Pennsylvania corporation, having a place of business at 1125 Trenton-Harbourton Road, Titusville, New Jersey 08560. 4. On information and belief, Defendant Amneal Pharms. LLC is a limited liability company organized under the laws of the State of Delaware and has a place of business at 400 Crossing Boulevard, Bridgewater, NJ 08807. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

Management Team
Management Team Bruce C. Cozadd Executive Chairman Bruce Cozadd joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Mr. Cozadd served as a consultant to companies in the biopharmaceutical industry. From 1991 until 2001, he held various positions with ALZA Corporation, a pharmaceutical company now owned by Johnson & Johnson, most recently as its Executive Vice President and Chief Operating Officer, with responsibility for research and development, manufacturing and sales and marketing. Previously at ALZA Corporation he held the roles of Chief Financial Officer and Vice President, Corporate Planning and Analysis. Mr. Cozadd received a B.S. from Yale University and an M.B.A. from the Stanford Graduate School of Business. Mr. Cozadd serves on the boards of Cerus Corporation, a biopharmaceutical company; Threshold Pharmaceuticals, a biotechnology company; and The Nueva School and Stanford Hospital and Clinics, both non-profit organizations. Samuel R. Saks, MD Chief Executive Officer Samuel Saks, M.D., joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Dr. Saks was Company Group Chairman of ALZA Corporation and served as a member of the Johnson & Johnson Pharmaceutical Group Operating Committee. From 1992 until 2001, he held various positions with ALZA Corporation, most recently as its Chief Medical Officer and Group Vice President, where he was responsible for clinical and commercial activities. Dr. Saks received a B.S. and an M.D. from the University of Illinois. Dr. Saks serves on the board of Trubion Pharmaceuticals and Cougar Biotechnology. Robert M. Myers President Robert Myers joined Jazz Pharmaceuticals at its inception and was appointed as Jazz Pharmaceuticals’ President in March 2007. -
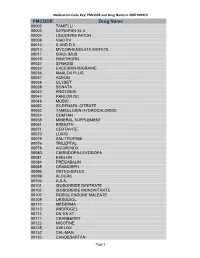
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

Frequently Asked Questions
Frequently Asked Questions Why are these companies included on the "Do Test" list? The following companies manufacture products that are tested on animals at some stage of development. Those marked with a Ƈ are currently observing a moratorium on (i.e., current suspension of) animal testing. Please encourage them to announce a permanent ban. Listed in parentheses are examples of products manufactured by either the company listed or, if applicable, its parent company. For a complete listing of products manufactured by a company on this list, please visit the company's website or contact the company directly for more information. Companies on this list may manufacture individual lines of products that have not been tested on animals. They have not, however, eliminated tests on animals for their entire line of cosmetics and household products. What if a company isn't on either of PETA's lists? Some companies have refused to respond to specific questions about their testing practices. It appears likely that these companies do test on animals at some stage of product development, and their refusal to clarify their testing policies appears to be an attempt to mislead consumers. Other companies may be new. If you find a company not included on our lists, please share the company's contact information with PETA so that we can contact the company directly. Legend Ƈ The company is currently observing a moratorium on animal testing. 3M 3M Corporate 1-888-364-3577 www.solutions.3m.com Headquarters 3M Center St. Paul, Minnesota 55144-1000 Acuvue (Johnson & Johnson) Customer (800) 843-2020 www.acuvue.com/ Relations, D-QA 7500 Centurion Parkway Jacksonville, Florida 32256 Aim (Church & Dwight) P.O.