(Myxozoa: Myxosporea) and the Spatial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ANALISIS ASPEK BIOLOGI IKAN TERBANG Cheilopogon Katoptron Bleeker, 1865, DI PERAIRAN PEMUTERAN, BALI BARAT TESIS DONY ARMA
UNIVERSITAS INDONESIA ANALISIS ASPEK BIOLOGI IKAN TERBANG Cheilopogon katoptron Bleeker, 1865, DI PERAIRAN PEMUTERAN, BALI BARAT TESIS DONY ARMANTO 0906577034 FAKULTAS MATEMATIKA DAN ILMU PENGETAHUAN ALAM PROGRAM MAGISTER ILMU KELAUTAN DEPOK JANUARI 2012 Analisis aspek..., Dony Armanto, FMIPA UI, 2012 2 UNIVERSITAS INDONESIA ANALISIS ASPEK BIOLOGI IKAN TERBANG Cheilopogon katoptron Bleeker, 1865, DI PERAIRAN PEMUTERAN, BALI BARAT TESIS Diajukan sebagai salah satu syarat untuk memperoleh gelar Magister Sains DONY ARMANTO 0906577034 FAKULTAS MATEMATIKA DAN ILMU PENGETAHUAN ALAM PROGRAM MAGISTER ILMU KELAUTAN DEPOK JANUARI 2012 ii Universitas Indonesia Analisis aspek..., Dony Armanto, FMIPA UI, 2012 3 HALAMAN PERNYATAAN ORISINALITAS Tesis ini adalah hasil karya sendiri, dan semua sumber baik yang dikutip maupun yang dirujuk telah saya nyatakan dengan benar. Nama : Dony Armanto NPM : 0906577034 Tanda Tangan : .............................. Tanggal : 3 Januari 2012 iii Universitas Indonesia Analisis aspek..., Dony Armanto, FMIPA UI, 2012 4 HALAMAN PENGESAHAN Tesis ini diajukan oleh: Nama : Dony Armanto NPM : 0906577034 Program Studi : Magister Ilmu Kelautan Judul Tesis : Analisis Aspek Biologi Ikan Terbang Cheilopogon katoptron Bleeker, 1865, di Perairan Pemuteran, Bali Barat Telah berhasil dipertahankan di hadapan Dewan Penguji dan diterima sebagai bagian persyaratan yang diperlukan untuk memperoleh gelar Magister Sains (M.Si) pada Program Studi Ilmu Kelautan, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Indonesia. DEWAN PENGUJI -

Review of Billfish Biology from Indian Fishery 1Bishnupadasethi and 2Ansy Mathew, N
IOTC–2014–WPB12–11 Rev_2 Review of Billfish biology from Indian fishery 1BishnupadaSethi and 2Ansy Mathew, N. P. 1Secretary & Commissioner (Fisheries), Government of Odisha, Bhubaneswar-751 001, Odisha, India. Email:[email protected] 2Fisheries Research and Investigation Officer, Department of Animal Husbandry, Dairying & Fisheries, Ministry of Agriculture, Government of India, KrishiBhawan, New Delhi-110114. E- mail: [email protected] Abstract In India, billfish fishery is contributed by Indo-pacific sailfish, blue marlin, black marlin, striped marlin and swordfish. The landings of the billfishes along the Indian coast are showing an increasing trend since the 1990s and the estimated landing during 2012 was 11613 t. Drift gillnets-cum-longline, handlines and longlines operated from mechanized and motorized craft contributed maximum to the catches. Along the east coast, peak catches occur during July- September and along the west coast during October-March. Length-weight structure and biology of the dominant species are presented and discussed. Keywords: Billfish, sailfish, drift gillnet, longline, by-catch Introduction In India, targeted fishery for billfishes does not exist, but this group constitute one of the most important components of bycatch in the longline, troll and oceanic drift gillnet fishery of Indian waters. Three species of marlins – stripped (Tetrapturus audax), blue (Makaira mazara) and black (M. Indica); Indo-Pacific sailfish (Istiophorus platypterus) and swordfish (Xiphias gladius) are the billfish species reported in the Indian fishery. The landings of the billfishes along the Indian coast are showing an increasing trend since the 1990s and the estimated landing during 2012 was 11613 t. Drift gillnets-cum-longline, handlines and longlines operated from mechanized and motorized craft contributed maximum to the catches. -

Ontogenetic and Seasonal Variations in the Feeding Ecology of Indo-Pacific Sailfish, Istiophorus Platypterus (Shaw, 1792), of the Eastern Arabian Sea
Indian Journal of Geo-Marine Sciences Vol. 42(5), September 2013, pp. 593-605 Ontogenetic and seasonal variations in the feeding ecology of Indo-Pacific sailfish, Istiophorus platypterus (Shaw, 1792), of the eastern Arabian Sea 1Sijo P. Varghese, 2V. S. Somvanshi & Deepak K. Gulati3 Cochin Base of Fishery Survey of India, PB No. 853, XIII/488, Kochangadi, Kochi 682005, India 2A - 1 Tower, Flat No. 701, Riddhi Gardens, Film City Road, Goregaon (East), Mumbai 400097, India Fishery Survey of India, Botawala Chambers, Sir P. M. Road, Mumbai 400001, India [Email: [email protected]] Received 21 May 2012; revised 21 August 2012 Present study consists the studies on the stomach contents of Indo-Pacific sailfish, Istiophorus platypterus (Shaw, 1792), caught during tuna longline survey conducted in the western Indian EEZ (eastern Arabian Sea) between 2006 and 2009 to investigate the sexual, ontogenetic and seasonal effects in the diet. Stomachs of 290 specimens in the forklength range of 101-261 cm were examined, of which 38 (13.10%) were empty. Prey composition was assessed in terms of occurrence by number, frequency of occurrence, weight and Index of Relative Importance. Quantile regression techniques were used to determine the mean and upper and lower bounds of the relation between prey size and sailfish length. Diet was dominated by teleost fishes, followed by cephalopods while crustaceans were represented in limited instances. Purpleback flying squid, Sthenoteuthis oualaniensis, was the most preferred prey species. Other important prey species identified were Euthynnus affinis, Cubiceps pauciradiatus, Gempylus serpens and Onychoteuthis banksii. Diet did not varied by sex, but the ontogenetic and seasonal variations in diet were significant. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -
Field Identification Guide to the Living Marine Resources in Kenya
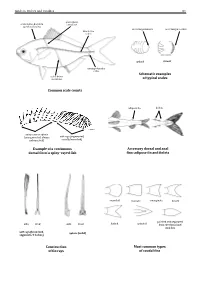
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S. -

The Intertidal Fish Fauna of the West Coast of South Africa - Species, Community and Biogeographic Patterns
S. Afr. 1. Zool. 1992.27(3) 115 The intertidal fish fauna of the west coast of South Africa - species, community and biogeographic patterns K. Prochazka * and C.L. Griffiths Zoology Department and Marine Biology Research Institute, University of Cape Town, Rondebosch, 7700 Republic of South Africa Received 5 November 1991 .. accepted 3 April 1992 In the first quantitative survey of intertidal fish from the South African west coast 62 intertidal rock pools were sampled at two sites, using the ichthyocide rotenone. A total of 2 022 fish representing 14 species belonging to only two families - the Clinidae (88-98% by number) and the Gobiesocidae (12-2%) - were caught. Clinus superciliosus, C. heterodon and the gobiesocid Chorisochismus dentex were the most abundant species in terms of both numbers and biomass. Vertical zonation of individual species on the shore indicated little separation of the habitat between species, although some species exhibited size-specific partitioning of the shore. Relationships between fish distribution and abundance and rock pool characteristics were elucidated by means of stepwise multiple regression, both at the whole community and individual species levels. The abundances of individual species were best predicted by pool size, although some species also showed an association with weed cover. For the community as a whole, the number of species present, the total number of fish and the total biomass in any pool were all dependent on pool size, height above LWS and amount of available cover. Relative to other South African sites the west coast has a low diversity of intertidal fish, combined with a high degree of dominance and a low level of habitat separation. -

Flyingfish by Robert Gillett and James Ianelli FFA Report 92/56
Flyingfish By Robert Gillett And James Ianelli FFA Report 92/56 PACIFIC ISLANDS FORUM FISHERIES AGENCY P.O.BOX 629 HONIARA SOLOMON ISLANDS TELEPHONE (677) 21124 FAX (677) 23995 WEB http://www.ffa.int CHAPTER 7 FLYINGFISH Robert Gillett and James Ianelli I. INTRODUCTION Flyingfish represent an important resource in many parts of the world. Several Pacific Islands currently have developed flyingfish fisheries and many have a history of traditional fisheries for flyingfish. Some Pacific islands do not have flyingfish fisheries, yet the abundance of the resource appears to be at least as great as other areas. As fishing pressure on limited reef resources increases, the development of alternative fisheries is needed, particularly for small- scale fishermen. Preliminary investigations suggest that flyingfish may also fall into this category. This chapter presents information obtained from a review of available literature, discussions with fisheries workers, correspondence with flyingfish authorities, and recent flyingfish fishing trials. This provides the basis for an assessment of the potential for fisheries development for this resource in the South Pacific. II. BIOLOGY In the following section, aspects of the biology of flyingfish are presented with as much reference as possible to the Pacific Island situation. In many cases, however, for lack of details specific to the Pacific Islands, information on studies from other parts of the world is provided. IDENTIFICATION In order to address fundamental questions on population dynamics and biology of flyingfish, it is important to be able to clearly identify the species involved. Flyingfishes (family Exocoetidae) are closely related to the garfishes (family Hemiramphidae), longtoms or needlefish (family Belonidae), and sauries (family Scomberosocidae). -

Genetic Variations of Cheilopogon Nigricans in the Makassar Strait, Indonesia
INDO PAC J OCEAN LIFE P-ISSN: 2775-1961 Volume 5, Number 1, June 2021 E-ISSN: 2775-1953 Pages: 22-28 DOI: 10.13057/oceanlife/o050104 Genetic variations of Cheilopogon nigricans in the Makassar Strait, Indonesia INDRAYANI INDRAYANI1,♥, MUHAMMAD NUR FINDRA1, ADY JUFRI2, HERLAN HIDAYAT3, ARMAN PARIAKAN4 1Faculty of Fisheries and Marine Science, Universitas Halu Oleo. Jl. H.E.A. Mokodopit, Kambu, Kendari 93561, Southeast Sulawesi, Indonesia. Tel.: +62-823-4267-8801, email: [email protected] 2Faculty of Animal Husbandry and Fisheries, Universitas Sulawesi Barat. Jl. Prof. Dr. Baharuddin Lopa, S.H., Lingkungan Talumung, Majene 91214, West Sulawesi, Indonesia 3Faculty of Forestry and Environmental Sciences, Universitas Halu Oleo. Jl. Syaikh Muhammad Al-Khidhir, Kambu, Kendari 93561, Southeast Sulawesi, Indonesia 4Faculty of Agriculture, Fisheries and Animal Husbandry, Universitas Sembilanbelas November. Jl. Pemuda, Tahoa, Kolaka 93561, Southeast Sulawesi, Indonesia Manuscript received: 23 April 2021. Revision accepted: 22 June 2021. Abstract. Indrayani I, Findra MN, Jufri A, Hidayat H, Pariakan A. 2021. Genetic variations of Cheilopogon nigricans in the Makassar Strait, Indonesia. Indo Pac J Ocean Life 5: 22-28. This study reports DNA Barcoding results (sequencing of cox 1 mitochondrial gene fragments) of four Makassar Strait flying fish species belonging to the Exocoetidae family. Sampling was collected from around the Makassar Strait waters in West Sulawesi. This research was carried out by molecular identification using DNA barcoding of the cytochrome oxidase 1 (COI) gene, the Wizard Promega CO1 primer kit. The molecular identification results showed that the collected fish had 100% and 99.10% genetic similarities with the species Cheilopogon nigricans from the South China Sea. -

Ward, A.B. and E. L. Brainerd. 2007. Evolution of Axial Patterning In
Blackwell Publishing LtdOxford, UKBIJBiological Journal of the Linnean Society0024-4066© 2006 The Linnean Society of London? 2006 90? 97116 Original Article AXIAL PATTERNING IN FISHES A. B. WARD and E. L. BRAINERD Biological Journal of the Linnean Society, 2007, 90, 97–116. With 9 figures Evolution of axial patterning in elongate fishes ANDREA B. WARD* and ELIZABETH L. BRAINERD† Biology Department and Organismic and Evolutionary Biology Program, University of Massachusetts, Amherst MA 01003, USA Received 7 July 2005; accepted for publication 1 March 2006 Within the ray-finned fishes, eel-like (extremely elongate) body forms have evolved multiple times from deeper-bod- ied forms. Previous studies have shown that elongation of the vertebral column may be associated with an increase in the number of vertebrae, an increase in the length of the vertebral centra, or a combination of both. Because the vertebral column of fishes has at least two anatomically distinct regions (i.e. abdominal and caudal), an increase in the number and relative length of the vertebrae could be region-specific or occur globally across the length of the ver- tebral column. In the present study, we recorded vertebral counts and measurements of vertebral aspect ratio (ver- tebral length/width) from museum specimens for 54 species representing seven groups of actinopterygian fishes. We also collected, from published literature, vertebral counts for 813 species from 14 orders of actinopterygian and elas- mobranch fishes. We found that the number of vertebrae can increase independently in the abdominal and caudal regions of the vertebral column, but changes in aspect ratio occur similarly in both regions. -

SPECIAL PUBLICATION No
The J. L. B. SMITH INSTITUTE OF ICHTHYOLOGY SPECIAL PUBLICATION No. 14 COMMON AND SCIENTIFIC NAMES OF THE FISHES OF SOUTHERN AFRICA PART I MARINE FISHES by Margaret M. Smith RHODES UNIVERSITY GRAHAMSTOWN, SOUTH AFRICA April 1975 COMMON AND SCIENTIFIC NAMES OF THE FISHES OF SOUTHERN AFRICA PART I MARINE FISHES by Margaret M. Smith INTRODUCTION In earlier times along South Africa’s 3 000 km coastline were numerous isolated communities. Interested in angling and pursuing commercial fishing on a small scale, the inhabitants gave names to the fishes that they caught. First, in 1652, came the Dutch Settlers who gave names of well-known European fishes to those that they found at the Cape. Names like STEENBRAS, KABELJOU, SNOEK, etc., are derived from these. Malay slaves and freemen from the East brought their names with them, and many were manufactured or adapted as the need arose. The Afrikaans names for the Cape fishes are relatively uniform. Only as the distance increases from the Cape — e.g. at Knysna, Plettenberg Bay and Port Elizabeth, do they exhibit alteration. The English names started in the Eastern Province and there are different names for the same fish at towns or holiday resorts sometimes not 50 km apart. It is therefore not unusual to find one English name in use at the Cape, another at Knysna, and another at Port Elizabeth differing from that at East London. The Transkeians use yet another name, and finally Natal has a name quite different from all the rest. The indigenous peoples of South Africa contributed practically no names to the fishes, as only the early Strandlopers were fish eaters and we know nothing of their language. -

Saldanha Bay and Langebaan Lagoon
State of the Bay 2006: Saldanha Bay and Langebaan Lagoon Technical Report Prepared by: L. Atkinson, K. Hutchings, B. Clark, J. Turpie, N. Steffani, T. Robinson & A. Duffell-Canham Prepared for: Saldanha Bay Water Quality Trust August 2006 Photograph by: Lyndon Metcalf Anchor Environmental Consultants CC STATE OF THE BAY 2006: SALDANHA BAY AND LANGEBAAN LAGOON TECHNICAL REPORT Prepared by: L. Atkinson, K. Hutchings, B. Clark, J. Turpie, N. Steffani, T. Robinson & A. Duffell-Canham AUGUST 2006 Prepared for: Copies of this report are available from: Anchor Environmental Consultants CC PO Box 34035, Rhodes Gift 7707 Tel/Fax: ++27-21-6853400 E-mail: [email protected] http://www.uct.ac.za/depts/zoology/anchor TABLE OF CONTENTS EXECUTIVE SUMMARY...........................................................................................................................................5 1 INTRODUCTION............................................................................................................................................10 2 STRUCTURE OF THIS REPORT..................................................................................................................13 3 BACKGROUND TO ENVIRONMENTAL MONITORING ..............................................................................14 3.1 MECHANISMS FOR MONITORING CONTAMINANTS AND THEIR EFFECTS ON THE ENVIRONMENT........................14 4 RANKING SYSTEM.......................................................................................................................................17