Saldanha Bay and Langebaan Lagoon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytoplankton Composition at Jeddah Coast–Red Sea, Saudi Arabia in Relation to Some Ecological Factors
JKAU: Sci., Vol. 22 No. 1, pp: 115-131 (2010 A.D. / 1431 A.H.); DOI: 10.4197 / Sci. 22-1.9 Phytoplankton Composition at Jeddah Coast–Red Sea, Saudi Arabia in Relation to some Ecological Factors Hussein E. Touliabah1, Wafaa S. Abu El-Kheir1 , Mohammed Gurban Kuchari2 and Najah Ibrahim Hassan Abdulwassi3 1 Botany Dept., Faculty of Girls, Ain Shams University, Cairo, 2 Egypt, Faculty Science, King Abdulaziz University, and 3 Faculty of Girls, King Abdulaziz University, Jeddah, Saudi Arabia. Abstract. Phytoplankton succession in relation to some physico- chemical characters of some water bodies at Jeddah Coast (Saudi Arabia) was studied for one year (2004). The sampling program included four different areas, North Obhour, Technology area, Down Town area and South Jeddah Area. Water samples were analyzed for some physico-chemical parameters (Temperature, pH, S‰, Dissolved +2 +2 Oxygen (DO), Calcium (Ca ) & Magnesium (Mg ), Nitrite (NO2), Nitrate (NO3), Ammonia (NH3), Reactive Orthophosphate (PO4) and Reactive Silicate (SiO3) as well as phycological parameters (Phytoplankton communities and Chlorophyll a). The Jeddah Coast was found to be oligotrophic ecosystem in some areas, while some of these areas were mesotrophic with high phytoplankton density such as the Down Town and South Jeddah areas. The results showed that, the high phytoplankton density attaining the maximum of 2623.2 X 103/m3 at Down Town area during spring and the minimum of 118.7 X 103/m3 at Technology area during winter. Seventy three species belonging to 73 genera and 5 groups were recorded. Dinophyceae was the first dominant group forming 43.8% of the total phytoplankton communities followed by Bacillariophyceae 27.9%. -
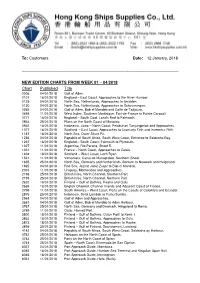
12 January, 2018 NEW EDITION CHARTS from WEEK 01
To: Customers Date: 12 January, 2018 _____________________________________________________________________________________________ NEW EDITION CHARTS FROM WEEK 01 – 04/2018 Chart Published Title 0006 04/01/2018 Gulf of Aden. 0107 18/01/2018 England – East Coast, Approaches to the River Humber. 0125 04/01/2018 North Sea, Netherlands, Approaches to Ijmuiden. 0130 04/01/2018 North Sea, Netherlands, Approaches to Scheveningen. 0265 04/01/2018 Gulf of Aden, Bab el Mandeb and Golfe de Tadjoura. 0494 11/01/2018 West Indies, Southern Martinique, Fort-de-France to Pointe Caracoli. 0777 18/01/2018 England – South Coat, Land’s End to Falmouth. 0863 25/01/2018 Plans on the North Coast of Morocco. 0932 18/01/2018 Indonesia, Jawa – North Coast, Pelabuhan Tanjungpriok and Approaches. 1077 18/01/2018 Scotland – East Coast, Approaches to Cromarty Firth and Inverness Firth. 1187 18/01/2018 North Sea, Outer Silver Pit. 1236 04/01/2018 Republic of South Africa, South West Coast, Entrance to Saldanha Bay. 1267 18/01/2018 England – South Coast, Falmouth to Plymouth. 1327 11/01/2018 Argentina, Rio Parana, Sheet 5. 1351 11/01/2018 France – North Coast, Approaches to Calais. 1404 18/01/2018 Scotland – West Coast, Loch Ryan. 1521 11/01/2018 Venezuela, Canal de Maraqcaibo, Southern Sheet. 1635 25/01/2018 North Sea, Germany and Netherlands, Borkum to Neuwerk and Helgoland. 1925 04/01/2018 Red Sea, Jazirat Jabal Zuqar to Bab el Mandeb. 2001 11/01/2018 Uruguay, Montevideo and Approaches. 2198 25/01/2018 British Isles, North Channel, Southern Part. 2199 25/01/2018 British Isles, North Channel, Northern Part. -

Flower Route Map 2014 LR
K o n k i e p en w R31 Lö Narubis Vredeshoop Gawachub R360 Grünau Karasburg Rosh Pinah R360 Ariamsvlei R32 e N14 ng Ora N10 Upington N10 IAi-IAis/Richtersveld Transfrontier Park Augrabies N14 e g Keimoes Kuboes n a Oranjemund r Flower Hotlines O H a ib R359 Holgat Kakamas Alexander Bay Nababeep N14 Nature Reserve R358 Groblershoop N8 N8 Or a For up-to-date information on where to see the Vioolsdrif nge H R27 VIEWING TIPS best owers, please call: Eksteenfontein a r t e b e e Namakwa +27 (0)79 294 7260 N7 i s Pella t Lekkersing t Brak u West Coast +27 (0)72 938 8186 o N10 Pofadder S R383 R383 Aggeneys Flower Hour i R382 Kenhardt To view the owers at their best, choose the hottest Steinkopf R363 Port Nolloth N14 Marydale time of the day, which is from 11h00 to 15h00. It’s the s in extended ower power hour. Respect the ower Tu McDougall’s Bay paradise: Walk with care and don’t trample plants R358 unnecessarily. Please don’t pick any buds, bulbs or N10 specimens, nor disturb any sensitive dune areas. Concordia R361 R355 Nababeep Okiep DISTANCE TABLE Prieska Goegap Nature Reserve Sun Run fels Molyneux Buf R355 Springbok R27 The owers always face the sun. Try and drive towards Nature Reserve Grootmis R355 the sun to enjoy nature’s dazzling display. When viewing Kleinzee Naries i R357 i owers on foot, stand with the sun behind your back. R361 Copperton Certain owers don’t open when it’s overcast. -

The Copyright of This Thesis Vests in the Author. No
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University THE EXPLOITATION OF FISH DURING THE HOLOCENE IN THE SOUTH- WESTERN CAPE, SOUTH AFRICA. Town CEDRIC ALAN POGGENPOELCape of Dissertation submitted in fulfilment of the requirements for a Master of Arts Degree in Archaeology University DEPARTMENT OF ARCHAEOLOGY UNIVERSITY OF CAPE TOWN 1996 \ •. :! I Town TO MY PARENTS RAY, JOHN AND GWENNIECape of University II ABSTRACT This thesis describes the fish remains recovered from a number of sites in three different localities in South Africa; Elands Bay and Langebaan Lagoon on the west coast, and False Bay on the Cape Peninsula. Chapter One is an introductory account of ichthyology, its usefulness in archaeological research and the range of analytical work done in South Africa, whilst Chapter Two is an attempt to show the history and development of the study of fish bones recovered from prehistoric sites in South Africa. Chapter Three gives an account of the southernTown Oceans, the Benguela Current and fishing habitats. Chapters Four and Five give accounts of the fishing habitats within the Elands Bay area and of the identification and interpretation of fish assemblages excavatedCape at four sites and their implications in relation to habitat and palaeoenvironmental changes at Elands Bay. -

Appendix A8 OPEN DAY ATTENDANCE REGISTERS
Transnet Limited Environmental Scoping Report Phase 2 Expansion: Port of Saldanha Appendix A8 OPEN DAY ATTENDANCE REGISTERS Prepared by the PDNA & SRK Joint Venture EIA for the Phase 2 Expansion of the Transnet Iron Ore Handling Facility, Saldanha Public Open Days, August 2006 Attendees Langebaan Library, Tuesday 15 August 2006: 1 Cannone, Al Dr. Vice-President Langebaan Ratepayers Association 2 Carruthers, H.G. Langebaan resident 3 Clemitson, Graeme Sir Saldanha Bay Forum 4 Genis, E.B. Bavaria Haus B&B 5 Halvorsen, Martin Director Cape West Coast Biosphere Reserve 6 Koen, Marius Councillor Saldanha Bay Local Municipality 7 Kruger, André Chairperson Saldanha Bay & Langebaan Tourism 8 Law, Kay Private Guest House Glenfinnan 9 MacKenzie, Binks Langebaan resident 10 Meissenheimer, H.D. Journalist Weslander 11 Steyn, Naudé Botanical Society of SA - West Coast 12 Steyn, W.C. Langebaan Ratepayers Association 13 Swart, C.H. Langebaan resident 14 Uys, Jannie Councillor Saldanha Bay Local Municipality 15 Van den Berg, Coenraad J. Chairman Langebaan Ratepayers Association 16 Walsh, Jimmy Director Cape West Coast Biosphere Reserve EIA / Project team: 1 Steenkamp, C. Transnet 2 Silomntu, M. SAPO 3 de Ruyter, P. SRK 4 Fourie, D. SRK 5 Woghiren, A. SEF 6 Aucamp, I. SEF Saldanha Protea Hotel, 15 August 2006 1 Arendse, Wilfred Saldanha resident 2 Butcher, Craig Projects Engineer SAPO 3 Camp, Ronel Court Official Department Correctional Services 4 Carnegie, Alan Saldanha resident 5 Davies, Ron Prof. UCT 6 Dickinson, Harry Projects Manager SAPO 7 Engelbrecht, Joey Saldanha resident 8 Fabricius, Piet Chairperson Saldanha Bay Forum 9 Fatuse, Headman White City resident 10 Geldenhuys, Insp. -

Joint Geological Survey/University of Cape Town MARINE GEOSCIENCE UNIT TECHNICAL ^REPORT NO. 13 PROGRESS REPORTS for the YEARS 1
Joint Geological Survey/University of Cape Town MARINE GEOSCIENCE UNIT TECHNICAL ^REPORT NO. 13 PROGRESS REPORTS FOR THE YEARS 1981-1982 Marine Geoscience Group Department of Geology University of Cape Town December 1982 NGU-Tfc—Kh JOINT GEOLOGICAL SURVEY/UNIVERSITY OF CAPE TOWN MARINE GEOSCIENCE UNIT TECHNICAL REPORT NO. 13 PROGRESS REPORTS FOR THE YEARS 1981-1982 Marine Geoscience Group Department of Geology University of Cape Town December 1982 The Joint Geological Survey/University of Cape Town Marine Geoscience Unit is jointly funded by the two parent organizations to promote marine geoscientific activity in South Africa. The Geological Survey Director, Mr L.N.J. Engelbrecht, and the University Research Committee are thanked for their continued generous financial and technical support for this work. The Unit was established in 1975 by the amalgamation of the Marine Geology Programme (funded by SANCOR until 1972) and the Marine Geophysical Unit. Financial ?nd technical assistance from the South African National Committee for Oceanographic Research, and the National Research Institute for Oceanology (Stellenbosch) are also gratefully acknowledged. It is the policy of the Geological Survey and the University of Cape Town that the data obtained may be presented in the form of theses for higher degrees and that completed projects shall be published without delay in appropriate media. The data and conclusions contained in this report are made available for the information of the international scientific community with tl~e request that they be not published in any manner without written permission. CONTENTS Page INTRODUCTION by R.V.Dingle i PRELIMINARY REPORT ON THE BATHYMETRY OF PART OF 1 THE TRANSKEI BASIN by S.H. -

A Paleoenvironmental Reconstruction of the Elands Bay Area Using Carbon and Nitrogen Isotopes in Torotoise Bone
A paleoenvironmental reconstruction of the Elands Bay area using carbon and nitrogen isotopes in tortoise bone. By Navashni Naidoo Supervised by Professor Judith Sealy Town Cape Dissertation submitted in fulfilmentof of the requirements for the degree of Master of Science (MSc) in Archaeology In the Department of Archaeology University of Cape Town UniversityJuly 2017 i The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Plagiarism Declaration I have used the Harvard convention for citation and referencing. Each contribution from, and quotation in, this thesis from the work(s) of other people has been attributed, and has been cited and referenced. This thesis is my own work. ii Abstract This study explores the utility of stable light isotopes in Chersina angulata (angulate or bowsprit tortoise) bone collagen as a paleoenvironmental proxy, to augment the limited range of proxies preserved in Southern Africa. δ13C and δ15N were measured in 76 archaeological tortoises from Elands Bay Cave and nearby Tortoise Cave. The samples range in age from the late Holocene to the terminal Pleistocene. δ15N values are not strongly correlated with δ13C, indicating different drivers of variation in the two isotopes. δ13C and δ15N values are lower between 154-487 cal. BP, which spans the Little Ice Age, compared with 744-1 042 cal. -

Economic State and Growth Prospects of the 12 Proclaimed Fishing Harbours in the Western Cape
Department of the Premier Report on the Economic and Socio- Economic State and Growth Prospects of the 12 Proclaimed Fishing Harbours in the Western Cape November 2012 Final consolidated report TABLE OF CONTENTS EXECUTIVE SUMMARY ............................................................................................ i 1 Introduction and purpose ................................................................................. 1 1.1 Project approach ..................................................................................................... 1 1.2 Project background ................................................................................................. 3 2 Policy and institutional context ........................................................................ 4 2.1 Historical context ..................................................................................................... 4 2.2 Local government context ....................................................................................... 4 2.3 Policy governing fishing harbours ........................................................................... 6 2.4 Key legislation relevant to fishing harbour governance............................................ 8 2.5 Previous investigations into harbour governance and management models to promote local socio-economic gains ..................................................................... 10 3 Assessment of fishing harbours and communities ...................................... 15 3.1 Introduction .......................................................................................................... -

Western Cape Biodiversity Spatial Plan Handbook 2017
WESTERN CAPE BIODIVERSITY SPATIAL PLAN HANDBOOK Drafted by: CapeNature Scientific Services Land Use Team Jonkershoek, Stellenbosch 2017 Editor: Ruida Pool-Stanvliet Contributing Authors: Alana Duffell-Canham, Genevieve Pence, Rhett Smart i Western Cape Biodiversity Spatial Plan Handbook 2017 Citation: Pool-Stanvliet, R., Duffell-Canham, A., Pence, G. & Smart, R. 2017. The Western Cape Biodiversity Spatial Plan Handbook. Stellenbosch: CapeNature. ACKNOWLEDGEMENTS The compilation of the Biodiversity Spatial Plan and Handbook has been a collective effort of the Scientific Services Section of CapeNature. We acknowledge the assistance of Benjamin Walton, Colin Fordham, Jeanne Gouws, Antoinette Veldtman, Martine Jordaan, Andrew Turner, Coral Birss, Alexis Olds, Kevin Shaw and Garth Mortimer. CapeNature’s Conservation Planning Scientist, Genevieve Pence, is thanked for conducting the spatial analyses and compiling the Biodiversity Spatial Plan Map datasets, with assistance from Scientific Service’s GIS Team members: Therese Forsyth, Cher-Lynn Petersen, Riki de Villiers, and Sheila Henning. Invaluable assistance was also provided by Jason Pretorius at the Department of Environmental Affairs and Development Planning, and Andrew Skowno and Leslie Powrie at the South African National Biodiversity Institute. Patricia Holmes and Amalia Pugnalin at the City of Cape Town are thanked for advice regarding the inclusion of the BioNet. We are very grateful to the South African National Biodiversity Institute for providing funding support through the GEF5 Programme towards layout and printing costs of the Handbook. We would like to acknowledge the Mpumalanga Biodiversity Sector Plan Steering Committee, specifically Mervyn Lotter, for granting permission to use the Mpumalanga Biodiversity Sector Plan Handbook as a blueprint for the Western Cape Biodiversity Spatial Plan Handbook. -

Synopsis of the Family Callianassidae, with Keys to Subfamilies, Genera and Species, and the Description of New Taxa (Crustacea: Decapoda: Thalassinidea)
ZV-326 (pp 03-152) 02-01-2007 14:37 Pagina 3 Synopsis of the family Callianassidae, with keys to subfamilies, genera and species, and the description of new taxa (Crustacea: Decapoda: Thalassinidea) K. Sakai Sakai, K. Synopsis of the family Callianassidae, with keys to subfamilies, genera and species, and the description of new taxa (Crustacea: Decapoda: Thalassinidea). Zool. Verh. Leiden 326, 30.vii.1999: 1-152, figs 1-33.— ISSN 0024-1652/ISBN 90-73239-72-9. K. Sakai, Shikoku University, 771-1192 Tokushima, Japan, e-mail: [email protected]. Key words: Crustacea; Decapoda; Thalassinidae; Callianassidae; synopsis. A synopsis of the family Callianassidae is presented. Defenitions are given of the subfamilies and genera. Keys to the sufamilies, genera, as well as seperate keys to the species occurring in certain bio- geographical areas are provided. At least the synonymy, type-locality, and distribution of the species are listed. The following new taxa are described: Calliapaguropinae subfamily nov., Podocallichirus genus nov., Callianassa whitei spec. nov., Callianassa gruneri spec. nov., Callianassa ngochoae spec. nov., Neocallichirus kempi spec. nov. and Calliax doerjesti spec. nov. Contents Introduction ............................................................................................................................. 3 Systematics .............................................................................................................................. 7 Subfamily Calliapaguropinae nov. ..................................................................................... -

Pyrenae 41-1 Pyrenae 04/06/10 10:33 Página 7
Pyrenae 41-1_Pyrenae 04/06/10 10:33 Página 7 PYRENAE, núm. 41, vol. 1 (2010) ISSN: 0079-8215 (p. 7-52) REVISTA DE PREHISTÒRIA I ANTIGUITAT DE LA MEDITERRÀNIA OCCIDENTAL JOURNAL OF WESTERN MEDITERRANEAN PREHISTORY AND ANTIQUITY Prehistoric Exploitation of Marine Resources in Southern Africa with Particular Reference to Shellfish Gathering: Opportunities and Continuities ANTONIETA JERARDINO Catalan Institution for Research and Advanced Studies (ICREA)/UB-GEPEG Department of Prehistory, Ancient History and Archaeology Universitat de Barcelona C/ Montalegre, 6-8, E-08001 Barcelona [email protected] This paper discusses three case studies in which marine resources played an important role in human development in southern Africa over the last 164 ka: the ability of modern humans to exit successfully from Africa is seen partly as the result of a foraging expansion from rocky shores to sandy beaches; the location of an aggregation site close to the coast in the context of low human densities during post-glacial times allowed people to meet social needs and ensure population survival; and a heavy reliance on marine resources supported highest populations levels during the late Holocene. Broader and related issues are also discussed. KEYWORDS COASTAL ARCHAEOLOGY, OUT OF AFRICA, AGGREGATION SITE, PLEISTOCENE/ HOLOCENE TRAN- SITION, RESOURCE INTENSIFICATION, MEGAMIDDENS. Este artículo se centra en la discusión de tres casos de estudio en los cuales los recursos marinos desempeñaron un rol importante en el desarrollo humano del sur de África desde hace 164 ka. La capacidad de los humanos modernos de emigrar fuera de África se ve, en parte, como una expansión en la capacidad de forrajeo inicialmente expresada en el litoral rocoso para incluir más tarde las costas de playas de arena. -

Sand Transport Along the Western Cape Coast
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Diazville Beach), and terrigenous-rich Sand transport along the Western beaches north ofprovided Shelley by Cape Point Town (upUniversity to OpenUCT 96 wt% terrigenous material in St Helena Cape coast: gone with the wind? Bay). The decrease in terrigenous material between Cape Town and Saldanha Bay a,b* a reflects the reduced delivery of terrigenous Giuliana Franceschini , John S. Compton and sand by rivers. False Bay and Table Bay a Rochelle A. Wigley have perennial rivers that drain catch- ment areas receiving an average annual rainfall of 600 mm. Ephemeral rivers with EACH SAND SAMPLES WERE COLLECTED Sixteen Mile Beach), carbonate-rich small catchment areas drain the semi-arid Bbetween Cape Town and St Helena Bay beaches between Saldanha Bay and west coast region south of the Berg River in order to study sediment composition Shelley Point (up to 83 wt% CaCO at (annual rainfall is 273 mm at Langebaan). and transport along the Western Cape coast- 3 line. Between Cape Town and Saldhana Bay, the beaches are a mixture of terrigenous and carbonate material. Those between Saldanha Bay and Shelley Point are carbonate-rich. North of Shelley Point, terrigenous-rich beaches were found. The decrease in terri- genous material from Cape Townto Saldanha Bay is a reflection of reduced delivery of terrigenous sand by rivers. The low content of terrigenous material in beach sands between Saldanha Bay and Shelley Point is related to the high biogenic CaCO3 in the rocky coastal area. In St Helena Bay the high percentage of terrigenous material is contributed by the Berg River.