A Paleoenvironmental Reconstruction of the Elands Bay Area Using Carbon and Nitrogen Isotopes in Torotoise Bone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cederberg Municipality Final Idp Review 2016/17
CEDERBERG MUNICIPALITY FINAL IDP REVIEW 2016/17 May 2016 Table of Contents EXECUTIVE MAYOR’S FOREWORD......................................................................................................... 6 MUNICIPAL MANAGER’S FOREWORD ................................................................................................... 7 CHAPTER 1 ......................................................................................................................................... 10 1.1. INTRODUCTION ................................................................................................................... 10 1.2. THE ROLE AND PURPOSE OF THE IDP ................................................................................... 10 1.3. LEGAL CONTEXT .................................................................................................................. 11 1.4. MUNICIPAL SNAPSHOT........................................................................................................ 12 1.5. Strategic Framework of the IDP ........................................................................................... 13 1.5.1. Vision and Mission ....................................................................................................... 13 ....................................................................................................................................................... 13 1.6. THE IDP PROCESS ............................................................................................................... -

Call to Join the Verlorenvlei Coalition
TUNGSTEN MINE THREATENS WAY OF LIFE OF THOUSANDS AND PLACES RAMSAR-SITE VERLORENVLEI AT HIGH RISK · We the people of the Verlorenvallei stand as one against a threat which could destroy our way of life and our valley. · We the farm workers, fishermen, farmers and entrepreneurs will not allow the pollution of our air, water or land or loss of our livelihoods for the sake of a greedy few. · We the lovers of nature reject further desecration of the already endangered Verlorenvlei and the unique and wide variety of animals, birds, reptiles and plants which have survived the depredations of humans. · We will protect the rare and largely unexplored rich pre-historical heritage for those who may follow us. · We have formed the Verlorenvlei Coalition; we are growing steadily, please join. The Verlorenvlei Coalition (VC) is a coalition of labour, business, civic organisations, environmental groups and local residents formed to preserve the integrity of the area and its people. We call our valley, which runs from Piketberg to Elands Bay, the Verlorenvallei. THE CHALLENGE: No less than 5 applicants have submitted applications to the DEPARTMENT OF MINING for the right to build an open-cast tungsten and molybdenum mine, one of these 50 hectares in extent and 200 metres deep, in the Moutonshoek Valley, between Piketberg and Elands Bay in the Western Cape. The Moutonshoek is a narrow valley, approximately 17 kilometres long and 3-4 kilometres wide, on the slopes of the Piketberg-mountain. THE VERLORENVLEI COALITION will oppose the proposed mining because: 1. It will destroy productive and profitable farms and detrimentally affect the food security of the Western Cape. -

Western Cape Provincial Crime Analysis Report 2015/16
Western Cape Provincial Crime Analysis Report 2015/16 Analysis of crime based on the 2015/16 crime statistics issued by the South African Police Service on the 2nd of September 2016 Department of Community Safety Provincial Secretariat for Safety and Security CONTENTS 1. INTRODUCTION AND CONTEXTUAL BACKGROUND 2 1.1 Limitations of crime statistics 2 2. METHODOLOGICAL APPROACH 3 3. KEY FINDINGS: 2014/15 - 2015/16 4 4. CONTACT CRIME ANALYSIS 6 4.1 Murder 6 4.2 Attempted murder 10 4.3 Total sexual crimes 13 4.4 Assault GBH 15 4.5 Common assault 18 4.6 Common robbery 20 4.7 Robbery with aggravating circumstances 22 4.8 Summary of violent crime in the Province 25 5. PROPERTY-RELATED CRIME 26 5.1 Burglary at non-residential premises 26 5.2 Burglary at residential premises 29 5.3 Theft of motor vehicle and motorcycle 32 5.4 Theft out of or from motor vehicle 34 5.5 Stock-theft 36 6. SUMMARY: 17 COMMUNITY-REPORTED SERIOUS CRIMES 37 6.1 17 Community-reported serious crimes 37 7. CRIME DETECTED AS A RESULT OF POLICE ACTION 38 7.1 Illegal possession of firearms and ammunition 38 7.2 Drug-related crime 43 7.3 Driving under the influence of alcohol or drugs 46 8. TRIO CRIMES 48 8.1 Car-jacking 48 8.2 Robbery at residential premises 50 8.3 Robbery at non-residential premises 53 9. CONCLUSION 56 Western Cape Provincial Crime Analysis Report 1 1. INTRODUCTION AND CONTEXTUAL BACKGROUND The South African Police Service (SAPS) annually releases reported and recorded crime statistics for the preceding financial year i.e. -

Two Holocene Rock Shelter Deposits from the Knersvlakte, Southern Namaqualand, South Africa
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers: part A Faculty of Science, Medicine and Health 1-1-2011 Two Holocene rock shelter deposits from the Knersvlakte, southern Namaqualand, South Africa Jayson Orton University of Cape Town Richard G. Klein Stanford University Alex Mackay Australian National University, [email protected] Steve E. Schwortz University of California - Davis Teresa E. Steele University of California - Davis Follow this and additional works at: https://ro.uow.edu.au/smhpapers Part of the Medicine and Health Sciences Commons, and the Social and Behavioral Sciences Commons Recommended Citation Orton, Jayson; Klein, Richard G.; Mackay, Alex; Schwortz, Steve E.; and Steele, Teresa E., "Two Holocene rock shelter deposits from the Knersvlakte, southern Namaqualand, South Africa" (2011). Faculty of Science, Medicine and Health - Papers: part A. 1762. https://ro.uow.edu.au/smhpapers/1762 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Two Holocene rock shelter deposits from the Knersvlakte, southern Namaqualand, South Africa Abstract This paper describes the first excavations into two Holocene Later Stone Age (LSA) deposits in southern Namaqualand. The limestone shelters afforded excellent preservation, and the LSA sites contained material similar in many respects to shelters in the Cederberg range to the south. Deposition at both sites was discontinuous with a mid-Holocene pulse in Buzz Shelter followed by contact-period deposits over a total depth of some 0.45 m. In Reception Shelter the 1.40 m deposit yielded a basal age in the fifth ot eighth centuries BC with pottery and domestic cow contained within a strong pulse of occupation just above this. -

Groundtruthing Establishing a Citizen Science
GROUNDTRUTHING ESTABLISHING A CITIZEN SCIENCE GROUNDWATER MONITORING NETWORK IN CAPE TOWN !1 © iStock Funder: This project was funded by WWF’s partner, AB InBev Author: GEOSS South Africa (Report number 2019/11-02) GEOSS South Africa is an earth science and groundwater consulting company that specialises in all aspects of groundwater development and management. geoss.co.za Reviewers: Julian Conrad (GEOSS), Dale Barrow (GEOSS), Klaudia Schachtschneider (WWF) Text editing: Marlene Rose Cover photo: © iStock Citation: WWF. 2020. Groundtruthing: Establishing a citizen science groundwater monitoring network in Cape Town. WWF South Africa, Cape Town, South Africa. © Text 2020 WWF South Africa If you would like to share copies of this paper, please do so in this printed or electronic PDF format. Available online at wwf.org.za/report/groundtruthing Published in 2020 by WWF – World Wide Fund for Nature (formerly World Wildlife Fund), Cape Town, South Africa. Any reproduction in full or in part must mention the title and credit the abovementioned publisher as the copyright owner. For more information, contact: Klaudia Schachtschneider Email: [email protected] or Email: [email protected] WWF is one of the world’s largest and most experienced independent conservation organisations with over 6 million supporters and a global network active in more than 100 countries. WWF’s mission is to stop the degradation of the planet’s natural environment and to build a future in which humans live in harmony with nature, by conserving the world’s biological diversity, ensuring that the use of renewable natural resources is sustainable, and promoting the reduction of pollution and wasteful consumption. -

Flower Route Map 2014 LR
K o n k i e p en w R31 Lö Narubis Vredeshoop Gawachub R360 Grünau Karasburg Rosh Pinah R360 Ariamsvlei R32 e N14 ng Ora N10 Upington N10 IAi-IAis/Richtersveld Transfrontier Park Augrabies N14 e g Keimoes Kuboes n a Oranjemund r Flower Hotlines O H a ib R359 Holgat Kakamas Alexander Bay Nababeep N14 Nature Reserve R358 Groblershoop N8 N8 Or a For up-to-date information on where to see the Vioolsdrif nge H R27 VIEWING TIPS best owers, please call: Eksteenfontein a r t e b e e Namakwa +27 (0)79 294 7260 N7 i s Pella t Lekkersing t Brak u West Coast +27 (0)72 938 8186 o N10 Pofadder S R383 R383 Aggeneys Flower Hour i R382 Kenhardt To view the owers at their best, choose the hottest Steinkopf R363 Port Nolloth N14 Marydale time of the day, which is from 11h00 to 15h00. It’s the s in extended ower power hour. Respect the ower Tu McDougall’s Bay paradise: Walk with care and don’t trample plants R358 unnecessarily. Please don’t pick any buds, bulbs or N10 specimens, nor disturb any sensitive dune areas. Concordia R361 R355 Nababeep Okiep DISTANCE TABLE Prieska Goegap Nature Reserve Sun Run fels Molyneux Buf R355 Springbok R27 The owers always face the sun. Try and drive towards Nature Reserve Grootmis R355 the sun to enjoy nature’s dazzling display. When viewing Kleinzee Naries i R357 i owers on foot, stand with the sun behind your back. R361 Copperton Certain owers don’t open when it’s overcast. -

Verlorenvlei Situation Assesment Final Draft Oct2009
C.A.P.E. Estuaries Programme DEVELOPMENT OF THE VERLORENVLEI ESTUARINE MANAGEMENT PLAN : ITUATION SSESSMENT S A (F INAL DRAFT ) October 2009 Report prepared for: Report prepared by: CapeNature CSIR West Coast District Municipality Natural Resources and the Department of Environmental Affairs Environment Department of Water Affairs Stellenbosch This report was compiled by: CSIR Natural Resources and the Environment PO Box 320 Stellenbosch 7599 Tel:+27 21 888-2400 Fax:+27 21 888-2693 Email: [email protected] This report should be referenced as: CSIR (2009) Development of the Verlorenvlei estuarine management plan: Situation assessment. Report prepared for the C.A.P.E. Estuaries Programme. CSIR Report No (to be allocated) Stellenbosch. COPYRIGHT © CSIR 2009 This document is copyright under the Berne Convention. In terms of the Copyright Act, Act No. 98 of 1978, no part of this book/document may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or by any information storage and retrieval system, without permission in writing from the CSIR. Cover Photos: P Huizinga and P Morant Final Draft: Verlorenvlei Situation Assessment Executive Summary Summary of Problems and Impacts Through stakeholder consultation, 16 key activities have been identified as threatening (or potentially threatening) to the ecosystem services provided by Verlorenvlei. These activities contribute to an array of problems and associated environmental impacts and socio-economic consequences, when managed inappropriately, as illustrated in Table 7.1 and Table 7.2. From these tables it is clear that there are no one-to-one linkages between the activities, problems and impacts – several activities can contribute to one or more problems which, in turn, can contribute to more than one impact, and vice versa. -

Cederberg-IDP May 2020 – Review 2020-2021
THIRD REVIEW: 2020/2021 MAY 2020 SECTIONS REVISED THIRD REVISION TO THE FOURTH GENERATION IDP ................... 0 3.8. INTERGOVERNMENTAL RELATIONS ................................. 67 FOREWORD BY THE EXECUTIVE MAYOR.................................. 2 3.9. INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) ...... 68 ACKNOWLEDGEMENT FROM THE MUNICIPAL MANAGER AND IMPORTANT MESSAGE ABOUT COVID-19 ................................. 4 CHAPTER 4: STRATEGIC OBJECTIVES AND PROJECT ALIGNMENT .. 71 EXECUTIVE SUMMARY ....................................................... 5 4.1 IMPROVE AND SUSTAIN BASIC SERVICE DELIVERY AND CHAPTER I: STATEMENT OF INTENT ...................................... 9 INFRASTRUCTURE .................................................... 73 1.1. INTRODUCTION ......................................................... 9 A. Water B. Electricity 1.2. THE FOURTH (4TH) GENERATION IDP .............................. 10 C. Sanitation D. Refuse removal / waste management 1.3. THE IDP AND AREA PLANS ........................................... 11 E. Roads F. Comprehensive Integrated Municipal Infrastructure Plan 1.4. POLICY AND LEGISLATIVE CONTEXT ................................ 11 G. Stormwater H. Integrated Infrastructure Asset Management Plan 1.5. STRATEGIC FRAMEWORK OF THE IDP .............................. 13 I. Municipal Infrastructure Growth Plan 1.6. VISION, MISSION, VALUES ............................................ 14 4.2 FINANCIAL VIABILITY AND ECONOMICALLY SUSTAINABILITY .... 87 1.7. STRATEGIC OBJECTIVES ............................................ -

Climatic Reconstruction Using Wood Charcoal from Archaeological Sites
CLIMATIC RECONSTRUCTION USING WOOD CHARCOAL FROM ARCHAEOLOGICAL SITES Town EDMUND CARL FEBRUARY Cape of Univesity Thesis presented to the University of Cape Town in fulfilment of the requirements for the degree of Master of Arts April 1990 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University ABSTRACT This thesis assesses the feasibility of using wood charcoal from archaeological sites as a palaeoclimatic indicator. Three techniques are described: (i) charcoal identification from Xylem Anatomy. (ii) Ecologically Diagnostic Xylem Analysis and (iii) stable carbon isotope analysis on wood charcoal. The first is a well established method of environmental reconstruction. This is the first systematic application of Ecologically Diagnostic Analysis and the first application of stable carbon isotope analysis on wood charcoal. Charcoal identification shows that the most common woody species at Elands Bay today are also evident in the archaeological record over the last 4000 years, indicating a relatively stable plant community composition. Previous studies of wood anatomy have shown that there are links between vessel size, vessel number and climate. This study demonstrates that the wood anatomy of Rhus is not simply related to climatic factors, necessitating the employment of a wide range of statistical analytical techniques to identify climatic signals. In contrast, the anatomy of Diospyros shows strong correlations with temperature. -

SLR CV Template
CURRICULUM VITAE ELOISE COSTANDIUS SENIOR ENVIRONMENTAL CONSULTANT Environmental Management, Planning & Approvals, South Africa QUALIFICATIONS Pr.Sci.Nat. 2010 Professional Natural Scientist (Environmental Science) with the South African Council for Natural Scientific Professions MSc 2005 Ecological Assessment BSc (Hons) 2002 Zoology BSc 2001 Biodiversity and Ecology, Botany, Zoology z EXPERTISE Eloise has worked as an environmental assessment practitioner since 2005 and has been involved in a number of projects covering a range of environmental Environmental Impact disciplines, including Basic Assessments, Environmental Impact Assessments, Assessment Environmental Management Programmes, Maintenance Management Plans, Public Participation Environmental Control Officer services and Public Consultation and Facilitation. In Environmental Auditing her 12 years as a consultant, she has gained experience in projects relating to oil Terrestrial Fauna and gas exploration, road infrastructure, renewable energy and housing and Assessment industrial developments. The majority of her work has been based in South Africa, but she also has experience working in Namibia and Mauritius. In addition, she has also undertaken a number of terrestrial fauna assessments as part of EIA specialist teams. PROJECTS Oil and Gas Spectrum Geo Ltd – Proposed EMP process for a Reconnaissance Permit Application to acquire 2D seismic data 2D Speculative Seismic Survey in a large area off the South-West and South coast of South Africa. Eloise was the off the South-West and South project manager and compiled the EMP report, undertook the required public Coast, South Africa (2018) participation process and managed the appointed specialists. Spectrum Geo Ltd - Proposed EMP process for a Reconnaissance Permit Application to acquire 2D seismic data 2D Speculative Seismic Survey within a large offshore area off the South and Southwest coast of South Africa. -

The Copyright of This Thesis Vests in the Author. No
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University THE EXPLOITATION OF FISH DURING THE HOLOCENE IN THE SOUTH- WESTERN CAPE, SOUTH AFRICA. Town CEDRIC ALAN POGGENPOELCape of Dissertation submitted in fulfilment of the requirements for a Master of Arts Degree in Archaeology University DEPARTMENT OF ARCHAEOLOGY UNIVERSITY OF CAPE TOWN 1996 \ •. :! I Town TO MY PARENTS RAY, JOHN AND GWENNIECape of University II ABSTRACT This thesis describes the fish remains recovered from a number of sites in three different localities in South Africa; Elands Bay and Langebaan Lagoon on the west coast, and False Bay on the Cape Peninsula. Chapter One is an introductory account of ichthyology, its usefulness in archaeological research and the range of analytical work done in South Africa, whilst Chapter Two is an attempt to show the history and development of the study of fish bones recovered from prehistoric sites in South Africa. Chapter Three gives an account of the southernTown Oceans, the Benguela Current and fishing habitats. Chapters Four and Five give accounts of the fishing habitats within the Elands Bay area and of the identification and interpretation of fish assemblages excavatedCape at four sites and their implications in relation to habitat and palaeoenvironmental changes at Elands Bay. -
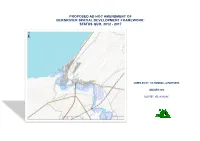
Proposed Ad Hoc Amendment of Bergrivier Spatial Development Framework: Status Quo, 2012 - 2017
PROPOSED AD HOC AMENDMENT OF BERGRIVIER SPATIAL DEVELOPMENT FRAMEWORK: STATUS QUO, 2012 - 2017 COMPILED BY: CK RUMBOLL & PARTNERS JANUARY 2018 OUR REF: VEL/10146/AC Contents 1. Purpose and approach .......................................................................................................................... 1 2. Detailed Status Quo Analysis and Implications .................................................................................... 3 2.1 Biophysical Environment ............................................................................................................... 3 2.2 Socio- Economic Environment .................................................................................................... 10 2.3 Built Environment ........................................................................................................................ 19 3. Strengths, Weaknesses, Opportunities and Threats (SWOT) ........................................................... 35 4. Recommendation ................................................................................................................................. 38 5. Maps illustrating Status Quo Analysis ................................................................................................ 39 List of Graphs Graph 1: Sectoral GDPR contribution (% share) to West Coast Economy (Quantec 2015 - MERO, 2017) .....................................................................................................................................................................