First Record of the Calanoid Copepod Pseudodiaptomus Serricaudatus (Scott, T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytoplankton Composition at Jeddah Coast–Red Sea, Saudi Arabia in Relation to Some Ecological Factors
JKAU: Sci., Vol. 22 No. 1, pp: 115-131 (2010 A.D. / 1431 A.H.); DOI: 10.4197 / Sci. 22-1.9 Phytoplankton Composition at Jeddah Coast–Red Sea, Saudi Arabia in Relation to some Ecological Factors Hussein E. Touliabah1, Wafaa S. Abu El-Kheir1 , Mohammed Gurban Kuchari2 and Najah Ibrahim Hassan Abdulwassi3 1 Botany Dept., Faculty of Girls, Ain Shams University, Cairo, 2 Egypt, Faculty Science, King Abdulaziz University, and 3 Faculty of Girls, King Abdulaziz University, Jeddah, Saudi Arabia. Abstract. Phytoplankton succession in relation to some physico- chemical characters of some water bodies at Jeddah Coast (Saudi Arabia) was studied for one year (2004). The sampling program included four different areas, North Obhour, Technology area, Down Town area and South Jeddah Area. Water samples were analyzed for some physico-chemical parameters (Temperature, pH, S‰, Dissolved +2 +2 Oxygen (DO), Calcium (Ca ) & Magnesium (Mg ), Nitrite (NO2), Nitrate (NO3), Ammonia (NH3), Reactive Orthophosphate (PO4) and Reactive Silicate (SiO3) as well as phycological parameters (Phytoplankton communities and Chlorophyll a). The Jeddah Coast was found to be oligotrophic ecosystem in some areas, while some of these areas were mesotrophic with high phytoplankton density such as the Down Town and South Jeddah areas. The results showed that, the high phytoplankton density attaining the maximum of 2623.2 X 103/m3 at Down Town area during spring and the minimum of 118.7 X 103/m3 at Technology area during winter. Seventy three species belonging to 73 genera and 5 groups were recorded. Dinophyceae was the first dominant group forming 43.8% of the total phytoplankton communities followed by Bacillariophyceae 27.9%. -
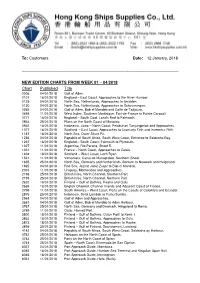
12 January, 2018 NEW EDITION CHARTS from WEEK 01
To: Customers Date: 12 January, 2018 _____________________________________________________________________________________________ NEW EDITION CHARTS FROM WEEK 01 – 04/2018 Chart Published Title 0006 04/01/2018 Gulf of Aden. 0107 18/01/2018 England – East Coast, Approaches to the River Humber. 0125 04/01/2018 North Sea, Netherlands, Approaches to Ijmuiden. 0130 04/01/2018 North Sea, Netherlands, Approaches to Scheveningen. 0265 04/01/2018 Gulf of Aden, Bab el Mandeb and Golfe de Tadjoura. 0494 11/01/2018 West Indies, Southern Martinique, Fort-de-France to Pointe Caracoli. 0777 18/01/2018 England – South Coat, Land’s End to Falmouth. 0863 25/01/2018 Plans on the North Coast of Morocco. 0932 18/01/2018 Indonesia, Jawa – North Coast, Pelabuhan Tanjungpriok and Approaches. 1077 18/01/2018 Scotland – East Coast, Approaches to Cromarty Firth and Inverness Firth. 1187 18/01/2018 North Sea, Outer Silver Pit. 1236 04/01/2018 Republic of South Africa, South West Coast, Entrance to Saldanha Bay. 1267 18/01/2018 England – South Coast, Falmouth to Plymouth. 1327 11/01/2018 Argentina, Rio Parana, Sheet 5. 1351 11/01/2018 France – North Coast, Approaches to Calais. 1404 18/01/2018 Scotland – West Coast, Loch Ryan. 1521 11/01/2018 Venezuela, Canal de Maraqcaibo, Southern Sheet. 1635 25/01/2018 North Sea, Germany and Netherlands, Borkum to Neuwerk and Helgoland. 1925 04/01/2018 Red Sea, Jazirat Jabal Zuqar to Bab el Mandeb. 2001 11/01/2018 Uruguay, Montevideo and Approaches. 2198 25/01/2018 British Isles, North Channel, Southern Part. 2199 25/01/2018 British Isles, North Channel, Northern Part. -

Joint Geological Survey/University of Cape Town MARINE GEOSCIENCE UNIT TECHNICAL ^REPORT NO. 13 PROGRESS REPORTS for the YEARS 1
Joint Geological Survey/University of Cape Town MARINE GEOSCIENCE UNIT TECHNICAL ^REPORT NO. 13 PROGRESS REPORTS FOR THE YEARS 1981-1982 Marine Geoscience Group Department of Geology University of Cape Town December 1982 NGU-Tfc—Kh JOINT GEOLOGICAL SURVEY/UNIVERSITY OF CAPE TOWN MARINE GEOSCIENCE UNIT TECHNICAL REPORT NO. 13 PROGRESS REPORTS FOR THE YEARS 1981-1982 Marine Geoscience Group Department of Geology University of Cape Town December 1982 The Joint Geological Survey/University of Cape Town Marine Geoscience Unit is jointly funded by the two parent organizations to promote marine geoscientific activity in South Africa. The Geological Survey Director, Mr L.N.J. Engelbrecht, and the University Research Committee are thanked for their continued generous financial and technical support for this work. The Unit was established in 1975 by the amalgamation of the Marine Geology Programme (funded by SANCOR until 1972) and the Marine Geophysical Unit. Financial ?nd technical assistance from the South African National Committee for Oceanographic Research, and the National Research Institute for Oceanology (Stellenbosch) are also gratefully acknowledged. It is the policy of the Geological Survey and the University of Cape Town that the data obtained may be presented in the form of theses for higher degrees and that completed projects shall be published without delay in appropriate media. The data and conclusions contained in this report are made available for the information of the international scientific community with tl~e request that they be not published in any manner without written permission. CONTENTS Page INTRODUCTION by R.V.Dingle i PRELIMINARY REPORT ON THE BATHYMETRY OF PART OF 1 THE TRANSKEI BASIN by S.H. -

Economic State and Growth Prospects of the 12 Proclaimed Fishing Harbours in the Western Cape
Department of the Premier Report on the Economic and Socio- Economic State and Growth Prospects of the 12 Proclaimed Fishing Harbours in the Western Cape November 2012 Final consolidated report TABLE OF CONTENTS EXECUTIVE SUMMARY ............................................................................................ i 1 Introduction and purpose ................................................................................. 1 1.1 Project approach ..................................................................................................... 1 1.2 Project background ................................................................................................. 3 2 Policy and institutional context ........................................................................ 4 2.1 Historical context ..................................................................................................... 4 2.2 Local government context ....................................................................................... 4 2.3 Policy governing fishing harbours ........................................................................... 6 2.4 Key legislation relevant to fishing harbour governance............................................ 8 2.5 Previous investigations into harbour governance and management models to promote local socio-economic gains ..................................................................... 10 3 Assessment of fishing harbours and communities ...................................... 15 3.1 Introduction .......................................................................................................... -

Synopsis of the Family Callianassidae, with Keys to Subfamilies, Genera and Species, and the Description of New Taxa (Crustacea: Decapoda: Thalassinidea)
ZV-326 (pp 03-152) 02-01-2007 14:37 Pagina 3 Synopsis of the family Callianassidae, with keys to subfamilies, genera and species, and the description of new taxa (Crustacea: Decapoda: Thalassinidea) K. Sakai Sakai, K. Synopsis of the family Callianassidae, with keys to subfamilies, genera and species, and the description of new taxa (Crustacea: Decapoda: Thalassinidea). Zool. Verh. Leiden 326, 30.vii.1999: 1-152, figs 1-33.— ISSN 0024-1652/ISBN 90-73239-72-9. K. Sakai, Shikoku University, 771-1192 Tokushima, Japan, e-mail: [email protected]. Key words: Crustacea; Decapoda; Thalassinidae; Callianassidae; synopsis. A synopsis of the family Callianassidae is presented. Defenitions are given of the subfamilies and genera. Keys to the sufamilies, genera, as well as seperate keys to the species occurring in certain bio- geographical areas are provided. At least the synonymy, type-locality, and distribution of the species are listed. The following new taxa are described: Calliapaguropinae subfamily nov., Podocallichirus genus nov., Callianassa whitei spec. nov., Callianassa gruneri spec. nov., Callianassa ngochoae spec. nov., Neocallichirus kempi spec. nov. and Calliax doerjesti spec. nov. Contents Introduction ............................................................................................................................. 3 Systematics .............................................................................................................................. 7 Subfamily Calliapaguropinae nov. ..................................................................................... -

Pyrenae 41-1 Pyrenae 04/06/10 10:33 Página 7
Pyrenae 41-1_Pyrenae 04/06/10 10:33 Página 7 PYRENAE, núm. 41, vol. 1 (2010) ISSN: 0079-8215 (p. 7-52) REVISTA DE PREHISTÒRIA I ANTIGUITAT DE LA MEDITERRÀNIA OCCIDENTAL JOURNAL OF WESTERN MEDITERRANEAN PREHISTORY AND ANTIQUITY Prehistoric Exploitation of Marine Resources in Southern Africa with Particular Reference to Shellfish Gathering: Opportunities and Continuities ANTONIETA JERARDINO Catalan Institution for Research and Advanced Studies (ICREA)/UB-GEPEG Department of Prehistory, Ancient History and Archaeology Universitat de Barcelona C/ Montalegre, 6-8, E-08001 Barcelona [email protected] This paper discusses three case studies in which marine resources played an important role in human development in southern Africa over the last 164 ka: the ability of modern humans to exit successfully from Africa is seen partly as the result of a foraging expansion from rocky shores to sandy beaches; the location of an aggregation site close to the coast in the context of low human densities during post-glacial times allowed people to meet social needs and ensure population survival; and a heavy reliance on marine resources supported highest populations levels during the late Holocene. Broader and related issues are also discussed. KEYWORDS COASTAL ARCHAEOLOGY, OUT OF AFRICA, AGGREGATION SITE, PLEISTOCENE/ HOLOCENE TRAN- SITION, RESOURCE INTENSIFICATION, MEGAMIDDENS. Este artículo se centra en la discusión de tres casos de estudio en los cuales los recursos marinos desempeñaron un rol importante en el desarrollo humano del sur de África desde hace 164 ka. La capacidad de los humanos modernos de emigrar fuera de África se ve, en parte, como una expansión en la capacidad de forrajeo inicialmente expresada en el litoral rocoso para incluir más tarde las costas de playas de arena. -

Sand Transport Along the Western Cape Coast
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Diazville Beach), and terrigenous-rich Sand transport along the Western beaches north ofprovided Shelley by Cape Point Town (upUniversity to OpenUCT 96 wt% terrigenous material in St Helena Cape coast: gone with the wind? Bay). The decrease in terrigenous material between Cape Town and Saldanha Bay a,b* a reflects the reduced delivery of terrigenous Giuliana Franceschini , John S. Compton and sand by rivers. False Bay and Table Bay a Rochelle A. Wigley have perennial rivers that drain catch- ment areas receiving an average annual rainfall of 600 mm. Ephemeral rivers with EACH SAND SAMPLES WERE COLLECTED Sixteen Mile Beach), carbonate-rich small catchment areas drain the semi-arid Bbetween Cape Town and St Helena Bay beaches between Saldanha Bay and west coast region south of the Berg River in order to study sediment composition Shelley Point (up to 83 wt% CaCO at (annual rainfall is 273 mm at Langebaan). and transport along the Western Cape coast- 3 line. Between Cape Town and Saldhana Bay, the beaches are a mixture of terrigenous and carbonate material. Those between Saldanha Bay and Shelley Point are carbonate-rich. North of Shelley Point, terrigenous-rich beaches were found. The decrease in terri- genous material from Cape Townto Saldanha Bay is a reflection of reduced delivery of terrigenous sand by rivers. The low content of terrigenous material in beach sands between Saldanha Bay and Shelley Point is related to the high biogenic CaCO3 in the rocky coastal area. In St Helena Bay the high percentage of terrigenous material is contributed by the Berg River. -

Towards Integrated Coastal Management for Saldanha Bay and Langebaan Lagoon, South Africa*
Africa Africa’ (1997)4 given me the opportunity totackle thisOceanography, issue. UCT, and tocomments the onFlemish a draft ofFundthis article.for I amScientific also indebtedResearch to Dr. Johnin Belgium, Largier of whothe Departmenthave of around the coast ofSouth Africa.3 One ofthese regions is the West Coast extensive processes ofinvolving interested and affectedformulation parties ofin regions a vision for the coast of South Africa identified through Environmental Affairs and Tourism and supported by the United Sowman of the Department of Environmental and Geographical Sciences, UCT, for their Sustainable Coastal set Developmentout South Act.2 Africa’s Kingdom’s futureIts Departmentpolicy for startingon International coastal Development. pointmanagement It is in supposedwas ato (CMPP). new the This programme was launched by South Africa’s productMinistry ofof an extensive process of public participation and specialist with the changing socio-political environment in South Africarevealed the studies carried out through the Coastal Management Policy Programme offact, the present Act only appliesto areas belowthe highwater mark. coastal development through integrated coastal management.1 As inadequacya matter of the existing Seashore Act (1935) to achieve sustainable point for virtually the whole spectrum ofhuman activities and is subjectto increasing development demands and urbanisation. This concern coupled The ecologically varied 3200-km South African coastal zone is the focal AFRICA* Jan Schrijvers** TOWARDS INTEGRATED COASTAL MANAGEMENT FOR 1 1 Introduction SALDANHA ANDBAY LANGEBAAN LAGOON, SOUTH 3 3 See generally CMPP 2 irism in March 1999. 1 1 See further J Glazewski ‘Towards a coastal zone management Act for South * A draft Coastal Policy White Paper was submitted in March 1999 as the as submitted by the Policy Committee to the Minister of Environmental Affairs and See generally CMPP MSc (Gent) PhD(Gent) postdoctoral researcherICZM, University ofGent, Belgium. -

NATO Maritime Interdiction Operational Training Center
Issue 11 2nd Issue 2015 ISSN: 2241-438X Maritime Interdiction Operations Journal TRAINING CENTER NATO MARITIME INTERDICTION OPERATIONAL NATO 1 NATO Maritime Interdiction Operational Training Center 7th Annual Conference CHALLENGES TO MARITIME SECURITY DERIVED FROM TRASNATIONAL ORGANISED CRIME AT SEA 07-09 JUNE 2016 2 C O N T E N T S COMMANDANT'S EDITORIAL TECHNOLOGICAL ISSUES MARITIME INTERDICTION OPERATIONS The Eastern and Central Editorial by Anastasios Tser Mediterranean Era of Oil JOURNAL 4 kezo glou, Commodore GRC 24 and Gas Importance for the (N) European Energy Security, by Anthony Foscolos, Emeritus Professor Director MARITIME SECURITY OPERATIONAL ISSUES Commodore Tserkezoglou GRC (N) Commandant NMIOTC Hybrid Threat, the New Understanding Risk in the 6 Evolving Challange to 31 Maritime Energy Security International Secu rity, by Domain, by Alexandra Hall Executive Director Corrado Campana, Ca ptain RAND, Europe ITA N Captain C. Campana ITA N Director of Training Support Maritime Terrorism the Threat Gulf of Guinea Maritime 8 to the National Security of 36 Criminality Analysis with the Middle East, by Sayed Geographic Informations Editor Chroneim, General (retired) Systems, by Miguel Bessa Lt Commander N. Tiantoukas GRC (N) Egyptian Army Pacheco, Cinav, Port N Head of Transformation Layout Production Protection Measures for Merchant Ships: Piracy Evi Sakellaridou 12 and Armed Robbery are a Maritime Energy Security HIGH VISIBILITY EVENTS Concern by Francesca De Rosa and Huw Davies, MSc MA, MBA FLNSTLM 44 VIP visitors to NMIOTC The views expressed in this LEGAL ISSUES NMIOTC TRAINING issue reflect the opinions of the authors, and do not nec- essarily represent NMIOTC's Contemporary Status of Mari Photos from NMIOTC or NATO’s official positions. -

COASTAL ENGINEERING in SOUTH AFRICA by K S RUSSELL 1. INTRODUCTION the Paper Presents a Review of the Historical Movement Of
COASTAL ENGINEERING IN SOUTH AFRICA by K S RUSSELL 1. INTRODUCTION The paper presents a review of the historical movement of ships around the South African coastline, traces the evolution and development of the harbours of South Africa, describes the development of coastal engineering and summarises the organisations and their activities in both basic and applied research projects contributing towards coastal works. 2. HISTORICAL The coastal currents and winds have played a major role in the historic exploration of the African coast. The earliest reference to a circum- navigation of Africa, although unconfirmed, was that by Herodotus who, in about 600 B.C. wrote that the Pharaoh Necho (Neco), then at war with the Syrians and wishing to combine his Mediterranean and Red Sea fleets, caused a fleet of ships manned by Phoenicians to sail from the Erythraean (Red) Sea and return through the Pillars of Hercules (Straits of Gibraltar). The journey is reputed to have taken three years; wind and currents make such a voyage in square-rigged boats a possibility. Fig,1. Ocean currents in the Southern hemisphere. The Restless Seas. Fig.2. The earliest navigations around Africa. Southern Land. A.R.Wilcox. National Research Institute for Oceanology, Stellenbosch, South Africa 2322 SOUTH AFRICA 2323 Accounts exist of voyages on the west coast by Hanno (c.500BC) who, with a fleet of 60 ships, explored as far as Cape Palmas (Liberia), and of Sataspes (c.475BC) who, in an attempt to sail around Libya (Africa) on the west and return by the Arabian Gulf (Red Sea), reached a similar destination. -

Class G Tables of Geographic Cutter Numbers: Maps -- by Region Or Country -- Eastern Hemisphere -- Africa
G8202 AFRICA. REGIONS, NATURAL FEATURES, ETC. G8202 .C5 Chad, Lake .N5 Nile River .N9 Nyasa, Lake .R8 Ruzizi River .S2 Sahara .S9 Sudan [Region] .T3 Tanganyika, Lake .T5 Tibesti Mountains .Z3 Zambezi River 2717 G8222 NORTH AFRICA. REGIONS, NATURAL FEATURES, G8222 ETC. .A8 Atlas Mountains 2718 G8232 MOROCCO. REGIONS, NATURAL FEATURES, ETC. G8232 .A5 Anti-Atlas Mountains .B3 Beni Amir .B4 Beni Mhammed .C5 Chaouia region .C6 Coasts .D7 Dra region .F48 Fezouata .G4 Gharb Plain .H5 High Atlas Mountains .I3 Ifni .K4 Kert Wadi .K82 Ktaoua .M5 Middle Atlas Mountains .M6 Mogador Bay .R5 Rif Mountains .S2 Sais Plain .S38 Sebou River .S4 Sehoul Forest .S59 Sidi Yahia az Za region .T2 Tafilalt .T27 Tangier, Bay of .T3 Tangier Peninsula .T47 Ternata .T6 Toubkal Mountain 2719 G8233 MOROCCO. PROVINCES G8233 .A2 Agadir .A3 Al-Homina .A4 Al-Jadida .B3 Beni-Mellal .F4 Fès .K6 Khouribga .K8 Ksar-es-Souk .M2 Marrakech .M4 Meknès .N2 Nador .O8 Ouarzazate .O9 Oujda .R2 Rabat .S2 Safi .S5 Settat .T2 Tangier Including the International Zone .T25 Tarfaya .T4 Taza .T5 Tetuan 2720 G8234 MOROCCO. CITIES AND TOWNS, ETC. G8234 .A2 Agadir .A3 Alcazarquivir .A5 Amizmiz .A7 Arzila .A75 Asilah .A8 Azemmour .A9 Azrou .B2 Ben Ahmet .B35 Ben Slimane .B37 Beni Mellal .B4 Berkane .B52 Berrechid .B6 Boujad .C3 Casablanca .C4 Ceuta .C5 Checkaouene [Tétouan] .D4 Demnate .E7 Erfond .E8 Essaouira .F3 Fedhala .F4 Fès .F5 Figurg .G8 Guercif .H3 Hajeb [Meknès] .H6 Hoceima .I3 Ifrane [Meknès] .J3 Jadida .K3 Kasba-Tadla .K37 Kelaa des Srarhna .K4 Kenitra .K43 Khenitra .K5 Khmissat .K6 Khouribga .L3 Larache .M2 Marrakech .M3 Mazagan .M38 Medina .M4 Meknès .M5 Melilla .M55 Midar .M7 Mogador .M75 Mohammedia .N3 Nador [Nador] .O7 Oued Zem .O9 Oujda .P4 Petitjean .P6 Port-Lyantey 2721 G8234 MOROCCO. -

Saldanha Bay and Langebaan Lagoon
State of the Bay 2006: Saldanha Bay and Langebaan Lagoon Technical Report Prepared by: L. Atkinson, K. Hutchings, B. Clark, J. Turpie, N. Steffani, T. Robinson & A. Duffell-Canham Prepared for: Saldanha Bay Water Quality Trust August 2006 Photograph by: Lyndon Metcalf Anchor Environmental Consultants CC STATE OF THE BAY 2006: SALDANHA BAY AND LANGEBAAN LAGOON TECHNICAL REPORT Prepared by: L. Atkinson, K. Hutchings, B. Clark, J. Turpie, N. Steffani, T. Robinson & A. Duffell-Canham AUGUST 2006 Prepared for: Copies of this report are available from: Anchor Environmental Consultants CC PO Box 34035, Rhodes Gift 7707 Tel/Fax: ++27-21-6853400 E-mail: [email protected] http://www.uct.ac.za/depts/zoology/anchor TABLE OF CONTENTS EXECUTIVE SUMMARY...........................................................................................................................................5 1 INTRODUCTION............................................................................................................................................10 2 STRUCTURE OF THIS REPORT..................................................................................................................13 3 BACKGROUND TO ENVIRONMENTAL MONITORING ..............................................................................14 3.1 MECHANISMS FOR MONITORING CONTAMINANTS AND THEIR EFFECTS ON THE ENVIRONMENT........................14 4 RANKING SYSTEM.......................................................................................................................................17