Introduction to Radioactivity and Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -
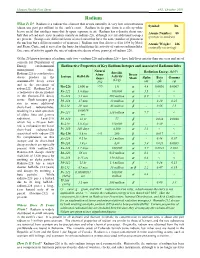
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Elementary Particles: an Introduction
Elementary Particles: An Introduction By Dr. Mahendra Singh Deptt. of Physics Brahmanand College, Kanpur What is Particle Physics? • Study the fundamental interactions and constituents of matter? • The Big Questions: – Where does mass come from? – Why is the universe made mostly of matter? – What is the missing mass in the Universe? – How did the Universe begin? Fundamental building blocks of which all matter is composed: Elementary Particles *Pre-1930s it was thought there were just four elementary particles electron proton neutron photon 1932 positron or anti-electron discovered, followed by many other particles (muon, pion etc) We will discover that the electron and photon are indeed fundamental, elementary particles, but protons and neutrons are made of even smaller elementary particles called quarks Four Fundamental Interactions Gravitational Electromagnetic Strong Weak Infinite Range Forces Finite Range Forces Exchange theory of forces suggests that to every force there will be a mediating particle(or exchange particle) Force Exchange Particle Gravitational Graviton Not detected so for EM Photon Strong Pi mesons Weak Intermediate vector bosons Range of a Force R = c Δt c: velocity of light Δt: life time of mediating particle Uncertainity relation: ΔE Δt=h/2p mc2 Δt=h/2p R=h/2pmc So R α 1/m If m=0, R→∞ As masses of graviton and photon are zero, range is infinite for gravitational and EM interactions Since pions and vector bosons have finite mass, strong and weak forces have finite range. Properties of Fundamental Interactions Interaction -

Neutrinoless Double Beta Decay
REPORT TO THE NUCLEAR SCIENCE ADVISORY COMMITTEE Neutrinoless Double Beta Decay NOVEMBER 18, 2015 NLDBD Report November 18, 2015 EXECUTIVE SUMMARY In March 2015, DOE and NSF charged NSAC Subcommittee on neutrinoless double beta decay (NLDBD) to provide additional guidance related to the development of next generation experimentation for this field. The new charge (Appendix A) requests a status report on the existing efforts in this subfield, along with an assessment of the necessary R&D required for each candidate technology before a future downselect. The Subcommittee membership was augmented to replace several members who were not able to continue in this phase (the present Subcommittee membership is attached as Appendix B). The Subcommittee solicited additional written input from the present worldwide collaborative efforts on double beta decay projects in order to collect the information necessary to address the new charge. An open meeting was held where these collaborations were invited to present material related to their current projects, conceptual designs for next generation experiments, and the critical R&D required before a potential down-select. We also heard presentations related to nuclear theory and the impact of future cosmological data on the subject of NLDBD. The Subcommittee presented its principal findings and comments in response to the March 2015 charge at the NSAC meeting in October 2015. The March 2015 charge requested the Subcommittee to: Assess the status of ongoing R&D for NLDBD candidate technology demonstrations for a possible future ton-scale NLDBD experiment. For each candidate technology demonstration, identify the major remaining R&D tasks needed ONLY to demonstrate downselect criteria, including the sensitivity goals, outlined in the NSAC report of May 2014. -

RIB Production by Photofission in the Framework of the ALTO Project
Available online at www.sciencedirect.com NIM B Beam Interactions with Materials & Atoms Nuclear Instruments and Methods in Physics Research B 266 (2008) 4092–4096 www.elsevier.com/locate/nimb RIB production by photofission in the framework of the ALTO project: First experimental measurements and Monte-Carlo simulations M. Cheikh Mhamed *, S. Essabaa, C. Lau, M. Lebois, B. Roussie`re, M. Ducourtieux, S. Franchoo, D. Guillemaud Mueller, F. Ibrahim, J.F. LeDu, J. Lesrel, A.C. Mueller, M. Raynaud, A. Said, D. Verney, S. Wurth Institut de Physique Nucle´aire, IN2P3-CNRS/Universite´ Paris-Sud, F-91406 Orsay Cedex, France Available online 11 June 2008 Abstract The ALTO facility (Acce´le´rateur Line´aire aupre`s du Tandem d’Orsay) has been built and is now under commissioning. The facility is intended for the production of low energy neutron-rich ion-beams by ISOL technique. This will open new perspectives in the study of nuclei very far from the valley of stability. Neutron-rich nuclei are produced by photofission in a thick uranium carbide target (UCx) using a 10 lA, 50 MeV electron beam. The target is the same as that already had been used on the previous deuteron based fission ISOL setup (PARRNE [F. Clapier et al., Phys. Rev. ST-AB (1998) 013501.]). The intended nominal fission rate is about 1011 fissions/s. We have studied the adequacy of a thick carbide uranium target to produce neutron-rich nuclei by photofission by means of Monte-Carlo simulations. We present the production rates in the target and after extraction and mass separation steps. -

Basics of Radiation Radiation Safety Orientation Open Source Booklet 1 (June 1, 2018)
Basics of Radiation Radiation Safety Orientation Open Source Booklet 1 (June 1, 2018) Before working with radioactive material, it is helpful to recall… Radiation is energy released from a source. • Light is a familiar example of energy traveling some distance from its source. We understand that a light bulb can remain in one place and the light can move toward us to be detected by our eyes. • The Electromagnetic Spectrum is the entire range of wavelengths or frequencies of electromagnetic radiation extending from gamma rays to the longest radio waves and includes visible light. Radioactive materials release energy with enough power to cause ionizations and are on the high end of the electromagnetic spectrum. • Although our bodies cannot sense ionizing radiation, it is helpful to think ionizing radiation behaves similarly to light. o Travels in straight lines with decreasing intensity farther away from the source o May be reflected off certain surfaces (but not all) o Absorbed when interacting with materials You will be using radioactive material that releases energy in the form of ionizing radiation. Knowing about the basics of radiation will help you understand how to work safely with radioactive material. What is “ionizing radiation”? • Ionizing radiation is energy with enough power to remove tightly bound electrons from the orbit of an atom, causing the atom to become charged or ionized. • The charged atoms can damage the internal structures of living cells. The material near the charged atom absorbs the energy causing chemical bonds to break. Are all radioactive materials the same? No, not all radioactive materials are the same. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Discovery of the Isotopes with Z<= 10
Discovery of the Isotopes with Z ≤ 10 M. Thoennessen∗ National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy, Michigan State University, East Lansing, MI 48824, USA Abstract A total of 126 isotopes with Z ≤ 10 have been identified to date. The discovery of these isotopes which includes the observation of unbound nuclei, is discussed. For each isotope a brief summary of the first refereed publication, including the production and identification method, is presented. arXiv:1009.2737v1 [nucl-ex] 14 Sep 2010 ∗Corresponding author. Email address: [email protected] (M. Thoennessen) Preprint submitted to Atomic Data and Nuclear Data Tables May 29, 2018 Contents 1. Introduction . 2 2. Discovery of Isotopes with Z ≤ 10........................................................................ 2 2.1. Z=0 ........................................................................................... 3 2.2. Hydrogen . 5 2.3. Helium .......................................................................................... 7 2.4. Lithium ......................................................................................... 9 2.5. Beryllium . 11 2.6. Boron ........................................................................................... 13 2.7. Carbon.......................................................................................... 15 2.8. Nitrogen . 18 2.9. Oxygen.......................................................................................... 21 2.10. Fluorine . 24 2.11. Neon........................................................................................... -

Chapter 5 the Relativistic Point Particle
Chapter 5 The Relativistic Point Particle To formulate the dynamics of a system we can write either the equations of motion, or alternatively, an action. In the case of the relativistic point par- ticle, it is rather easy to write the equations of motion. But the action is so physical and geometrical that it is worth pursuing in its own right. More importantly, while it is difficult to guess the equations of motion for the rela- tivistic string, the action is a natural generalization of the relativistic particle action that we will study in this chapter. We conclude with a discussion of the charged relativistic particle. 5.1 Action for a relativistic point particle How can we find the action S that governs the dynamics of a free relativis- tic particle? To get started we first think about units. The action is the Lagrangian integrated over time, so the units of action are just the units of the Lagrangian multiplied by the units of time. The Lagrangian has units of energy, so the units of action are L2 ML2 [S]=M T = . (5.1.1) T 2 T Recall that the action Snr for a free non-relativistic particle is given by the time integral of the kinetic energy: 1 dx S = mv2(t) dt , v2 ≡ v · v, v = . (5.1.2) nr 2 dt 105 106 CHAPTER 5. THE RELATIVISTIC POINT PARTICLE The equation of motion following by Hamilton’s principle is dv =0. (5.1.3) dt The free particle moves with constant velocity and that is the end of the story. -

1.1. Introduction the Phenomenon of Positron Annihilation Spectroscopy
PRINCIPLES OF POSITRON ANNIHILATION Chapter-1 __________________________________________________________________________________________ 1.1. Introduction The phenomenon of positron annihilation spectroscopy (PAS) has been utilized as nuclear method to probe a variety of material properties as well as to research problems in solid state physics. The field of solid state investigation with positrons started in the early fifties, when it was recognized that information could be obtained about the properties of solids by studying the annihilation of a positron and an electron as given by Dumond et al. [1] and Bendetti and Roichings [2]. In particular, the discovery of the interaction of positrons with defects in crystal solids by Mckenize et al. [3] has given a strong impetus to a further elaboration of the PAS. Currently, PAS is amongst the best nuclear methods, and its most recent developments are documented in the proceedings of the latest positron annihilation conferences [4-8]. PAS is successfully applied for the investigation of electron characteristics and defect structures present in materials, magnetic structures of solids, plastic deformation at low and high temperature, and phase transformations in alloys, semiconductors, polymers, porous material, etc. Its applications extend from advanced problems of solid state physics and materials science to industrial use. It is also widely used in chemistry, biology, and medicine (e.g. locating tumors). As the process of measurement does not mostly influence the properties of the investigated sample, PAS is a non-destructive testing approach that allows the subsequent study of a sample by other methods. As experimental equipment for many applications, PAS is commercially produced and is relatively cheap, thus, increasingly more research laboratories are using PAS for basic research, diagnostics of machine parts working in hard conditions, and for characterization of high-tech materials. -

Chapter 3 the Fundamentals of Nuclear Physics Outline Natural
Outline Chapter 3 The Fundamentals of Nuclear • Terms: activity, half life, average life • Nuclear disintegration schemes Physics • Parent-daughter relationships Radiation Dosimetry I • Activation of isotopes Text: H.E Johns and J.R. Cunningham, The physics of radiology, 4th ed. http://www.utoledo.edu/med/depts/radther Natural radioactivity Activity • Activity – number of disintegrations per unit time; • Particles inside a nucleus are in constant motion; directly proportional to the number of atoms can escape if acquire enough energy present • Most lighter atoms with Z<82 (lead) have at least N Average one stable isotope t / ta A N N0e lifetime • All atoms with Z > 82 are radioactive and t disintegrate until a stable isotope is formed ta= 1.44 th • Artificial radioactivity: nucleus can be made A N e0.693t / th A 2t / th unstable upon bombardment with neutrons, high 0 0 Half-life energy protons, etc. • Units: Bq = 1/s, Ci=3.7x 1010 Bq Activity Activity Emitted radiation 1 Example 1 Example 1A • A prostate implant has a half-life of 17 days. • A prostate implant has a half-life of 17 days. If the What percent of the dose is delivered in the first initial dose rate is 10cGy/h, what is the total dose day? N N delivered? t /th t 2 or e Dtotal D0tavg N0 N0 A. 0.5 A. 9 0.693t 0.693t B. 2 t /th 1/17 t 2 2 0.96 B. 29 D D e th dt D h e th C. 4 total 0 0 0.693 0.693t /th 0.6931/17 C. -

A Measurement of the 2 Neutrino Double Beta Decay Rate of 130Te in the CUORICINO Experiment by Laura Katherine Kogler
A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Physics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Stuart J. Freedman, Chair Professor Yury G. Kolomensky Professor Eric B. Norman Fall 2011 A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment Copyright 2011 by Laura Katherine Kogler 1 Abstract A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler Doctor of Philosophy in Physics University of California, Berkeley Professor Stuart J. Freedman, Chair CUORICINO was a cryogenic bolometer experiment designed to search for neutrinoless double beta decay and other rare processes, including double beta decay with two neutrinos (2νββ). The experiment was located at Laboratori Nazionali del Gran Sasso and ran for a period of about 5 years, from 2003 to 2008. The detector consisted of an array of 62 TeO2 crystals arranged in a tower and operated at a temperature of ∼10 mK. Events depositing energy in the detectors, such as radioactive decays or impinging particles, produced thermal pulses in the crystals which were read out using sensitive thermistors. The experiment included 4 enriched crystals, 2 enriched with 130Te and 2 with 128Te, in order to aid in the measurement of the 2νββ rate. The enriched crystals contained a total of ∼350 g 130Te. The 128-enriched (130-depleted) crystals were used as background monitors, so that the shared backgrounds could be subtracted from the energy spectrum of the 130- enriched crystals.