Current Status of Granulocyte–Macrophage Colony-Stimulating
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lenograstim in the Treatment of Severe Neutropenia in Patients Treated with Peg-IFN and Ribavirin
Le Infezioni in Medicina, n. 1, 21-23, 2009 Lavori originali Lenograstim in the treatment of severe neutropenia in Original articles patients treated with Peg-IFN and ribavirin: the experience of a single hepatology unit Trattamento mediante lenograstim della neutropenia grave in corso di terapia con Peg-IFN e ribavirina: esperienza di una singola unità di epatologia Luciano Tarantino1, Annunziata De Rosa2, Orsola Tambaro1, Carmine Ripa1, Marta Celiento3, Antonio Schiano1 1Unità di Epatologia ed Ecointerventistica, Ospedale San Giovanni di Dio, Frattamaggiore, Napoli, Italy; 2AOU “Federico II”, Dipartimento di Malattie Infettive, ASL NA 2, Napoli, Italy; 3AOU “Federico II”, Dipartimento di Chirurgia Generale e Geriatria, ASL NA 2, Napoli, Italy n INTRODUCTION doses of 80 mcg s.c./week of Peg-IFN 2b and 1200 mg/die of ribavirin. We checked quanti- he standard treatment of chronic hepatitis C tative HCV- RNA at 12 and 48 weeks of treat- (ECA C) is pegylated interferon-alpha (Peg- ment. Every two weeks, two days before ad- TIFN ) combined with ribavirin [1]. One of the ministration of the weekly dose of Peg-IFN 2b most frequent causes of suspension of therapy or all patients had blood count and liver enzyme reduction of the doses of Peg-IFN is haemato- tests. Patients with absolute neutrophil counts toxicity, which includes anaemia and/or neu- <900 cells/mmc started on lenograstim (263 tropenia and/or thrombocytopenia [2]. The use µg) 24 hours before administration of Peg- of growth factors (G-CSF) in responder patients IFNα 2b (Figure 1). Indication for administer- allows effective doses of Peg-IFN and RIBA to be ing lenograstim was early viral response kept stable [3]. -

Pegasys, INN-Peginterferon Alfa-2A
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion and scientific discussion on procedures, which have been finalised before 1 April 2005. For scientific information on procedures after this date please refer to module 8B. 1. Introduction Peginterferon alfa-2a is a polyethylene glycol (PEG)-modified form of human recombinant interferon alfa-2a intended for the treatment of adult patients with chronic hepatitis C (CHC) or chronic hepatitis B (CHB). Chronic hepatitis C is a major public health problem: hepatitis C virus (HCV) is responsible for a large proportion of chronic liver disease, accounting for 70% of cases of chronic hepatitis in industrialised countries. Globally there are an estimated 150 million chronic carriers of the virus, including 5 million in Western Europe. Without treatment approximately 30% of those infected with HCV will develop cirrhosis over a time frame of 30 years or more. For those with HCV-related cirrhosis, the prognosis is poor – a significant proportion will develop a life-threatening complication (either decompensated liver disease or an hepatocellular carcinoma) within a few years. The only therapy for those with advanced cirrhosis is liver transplantation, which carries a high mortality. In those who survive transplantation, viral recurrence in the new liver is almost inevitable and a significant proportion of infected liver grafts develop a progressive fibrosis that leads to recurrence of cirrhosis within 5 years. Interferon alfa monotherapy has been shown to be effective for the treatment of chronic hepatitis although sustained response rates occurred in approximately 15 to 30 % of patients treated for long duration (12-18 months). The current reference therapy is interferon alpha in combination with ribavirin, which resulted in an increase in biochemical and virological sustained response rates to approximately 40 % in naïve patients. -

Alfa Interferons
Clinical Pharmacy Program Guidelines for Alfa Interferons Program Prior Authorization Medication Intron A (interferon alfa-2b), Pegasys (peginterferon alfa-2a), PegIntron and Sylatron™ (peginterferon alfa-2b) Markets in Scope California,Colorado,Hawaii, Maryland, Nevada, New Jersey, New York, New York EPP, Pennsylvania- CHIP, Rhode Island, South Carolina Issue Date 9/2009 Pharmacy and 11/2020 Therapeutics Approval Date Effective Date 12/2020 1. Background: Indications Intron A (interferon alfa-2b) is indicated for the treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease who have a history of blood or blood- product exposure and/or are HCV antibody positive. Intron A has additional FDA labeling for the treatment of chronic hepatitis C in patients 3 years of age and older with compensated liver disease previously untreated with alpha interferon therapy and in patients 18 years of age and older who have relapsed following alpha interferon therapy. Intron A is also indicated for the treatment of chronic hepatitis B in patients 1 year of age or older with compensated liver disease. Patients who have been serum HBsAg positive for at least 6 months and have evidence of HBV replication (serum HBeAg positive) with elevated serum ALT are candidates for treatment. Intron A is indicated for the treatment of patients 18 years of age or older with hairy cell leukemia. Intron A is indicated as adjuvant to surgical treatment in patients 18 years of age or older with malignant melanoma who are free of disease but a high risk for systemic recurrence, within 56 days of surgery. -
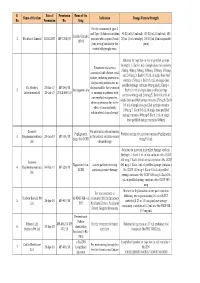
Final List of R-DNA Based Drugs Approved in the Country.Xlsx
S. Date of Permission Name of the Name of the firm Indication Dosage Form & Strength No. Permission No. Drug For the treatment of type -I and Type -II diabetes mellitus 40 IU/ml (10 ml vial), 100 IU/ml (10 ml vial), 100 Insulin Glargine 1 Wockhardt Limited 22.02.2007 MF-7206/07 patients who required basal IU/ml (3 ml cartridge), 100 IU/ml (3 ml disposable 100 IU (long acting) insulin for the pens) control of hyperglycemia. Solution for injection in vial or prefilled syringe Strength: 1. Each 1 mL of single dose vial contains Treatment of anaemia 25mcg, 40mcg, 60mcg, 100mcg, 200mcg, 300mcg associated with chronic renal and 500mcg 2. Each 0.75 mL of single dose vial failure, including patients on contains 150mcg 3. Each 0.3 mL of single dose dialysis and patients not on prefilled syringe contains 60mcg and 150mcg 4. Dr. Reddy's 23-Mar-10 MF-246/10 dialysis and for the treatment 2 Darbepoetin alfa Each 0.4 mL of single dose prefilled syringe Laboratories Ltd. 20-Jul-10 BULK-668/10 of anaemia in patients with contains 40mcg and 200mcg 5. Each 0.42 mL of non-myeloid malignancies, single dose prefilled syringe contains 25mcg 6. Each where anaemia is due to the 0.5 mL of single dose prefilled syringe contains effect of concomitantly 100mcg 7. Each 0.6 mL of single dose prefilled administered chemotherapy syringe contains 300mcg 8. Each 1 mL of single dose prefilled syringe contains 500mcg Gennova For prevention of neutropenia Pegfilgrastim Prefilled syringe for injection contains Pegfilgrastim 3 Biopharmaceuticals 29-Jan-10 MF-104/10 in the patient receiving cancer (peg-r-hu-GCSF) 6 mg/0.6 mL Ltd. -

Use of Interferon Alfa in the Treatment of Myeloproliferative Neoplasms: Perspectives and Review of the Literature
cancers Review Use of Interferon Alfa in the Treatment of Myeloproliferative Neoplasms: Perspectives and Review of the Literature Joan How 1,2,3 and Gabriela Hobbs 1,* 1 Department of Medical Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; [email protected] 2 Division of Hematology, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA 3 Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115, USA * Correspondence: [email protected]; Tel.: +1-617-724-1124 Received: 26 June 2020; Accepted: 10 July 2020; Published: 18 July 2020 Abstract: Interferon alfa was first used in the treatment of myeloproliferative neoplasms (MPNs) over 30 years ago. However, its initial use was hampered by its side effect profile and lack of official regulatory approval for MPN treatment. Recently, there has been renewed interest in the use of interferon in MPNs, given its potential disease-modifying effects, with associated molecular and histopathological responses. The development of pegylated formulations and, more recently, ropeginterferon alfa-2b has resulted in improved tolerability and further expansion of interferon’s use. We review the evolving clinical use of interferon in essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF). We discuss interferon’s place in MPN treatment in the context of the most recent clinical trial results evaluating interferon and its pegylated formulations, and its role in special populations such as young and pregnant MPN patients. Interferon has re-emerged as an important option in MPN patients, with future studies seeking to re-establish its place in the existing treatment algorithm for MPN, and potentially expanding its use for novel indications and combination therapies. -

INTRON A, Interferon Alfa-2B
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT IntronA 3 million IU/0.5 mL solution for injection or infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial of solution for injection or infusion contains 3 million IU of recombinant interferon alfa-2b produced in E. coli by recombinant DNA technology, in 0.5 mL of solution. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection or infusion. Clear and colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Chronic hepatitis B Treatment of adult patients with chronic hepatitis B associated with evidence of hepatitis B viral replication (presence of DNA of hepatitis B virus (HBV-DNA) and hepatitis B antigen (HBeAg), elevated alanine aminotransferase (ALT) and histologically proven active liver inflammation and/or fibrosis. Chronic hepatitis C Before initiating treatment with IntronA, consideration should be given to the results from clinical trials comparing IntronA with pegylated interferon (see section 5.1). Adult patients IntronA is indicated for the treatment of adult patients with chronic hepatitis C who have elevated transaminases without liver decompensation and who are positive for hepatitis C virus RNA (HCV-RNA) (see section 4.4). The best way to use IntronA in this indication is in combination with ribavirin. Children 3 years of age and older and adolescents IntronA is indicated, in a combination regimen with ribavirin, for the treatment of children 3 years of age and older and adolescents, who have chronic hepatitis C, not previously treated, without liver decompensation, and who are positive for HCV-RNA. -

Diagnosis Code-Restricted Physician-Administered Drugs
Diagnosis Code-Restricted Physician-Administered Drugs The following table contains diagnosis-restricted physician-administered drugs and the corresponding diagnosis code and disease descriptions. When a physician-administered drug claim is submitted with a diagnosis listed in this attachment, prior authorization (PA) is not required. For uses outside the listed diagnosis, PA is required. Submission of peer-reviewed medical literature from scientific medical or pharmaceutical publications in which original manuscripts are rejected or published only after having been reviewed by unbiased independent experts to support the proven efficacy of the requested use of the drug is also required to be submitted with the PA request. Note: This table includes Wisconsin Medicaid’s most current information and may be updated periodically. Effective: July 1, 2012 HCPCSCode* Drug Name Diagnosis Code Disease Description J0205 Alglucerase (Ceredase) 2727 Gaucher’s Disease 3336 Idiopathic dystonia 3337 Symptomatic torsion dystonia 33381 Blepharospasm 33383 Spasmodic torticollis 33384 Focal hand dystonia Spastic hemiplegia and hemiparesis affecting 34211 dominant side Spastic hemiplegia and hemiparesis affecting 34212 Botulinum Toxin Type A nondominant side J0585 (Botox) 3440-34404, 34409 Quadriplegia 3441 Paraplegia 340 Multiple Sclerosis 3430-3439 Cerebral palsy 3518 Facial spasm 3780-37887 Strabismus 70521 Hyperhidrosis 72885 Spasm of muscle 7810 Hemifacial spasm Botulinum Toxin Type B J0587 33383 Spasmodic torticollis (Myobloc) J0740 Cidofovir (Vistide), -

International Nonproprietary Names (Inn) for Biological and Biotechnological Substances
INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 15/06/2006 INTERNATIONAL NONPROPRIETARY NAMES (INN) FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES (A REVIEW) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines (QSM) Medicines Policy and Standards (PSM) Department CONTENTS 0. INTRODUCTION…………………………………….........................................................................................v 1. PHARMACOLOGICAL CLASSIFICATION OF BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………................................1 2. CURRENT STATUS OF EXISTING STEMS OR SYSTEMS FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES…………………….3 2.1 Groups with respective stems ……………………………………………………………………3 2.2 Groups with respective pre-stems………………………………………………………………4 2.3 Groups with INN schemes………………………………………………………………………….4 2.4 Groups without respective stems / pre-stems and without INN schemes…..4 3. GENERAL POLICIES FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………………………………………………………………………...5 3.1 General policies for blood products……………………………………………………………5 3.2 General policies for fusion proteins……………………………………………………………5 3.3 General policies for gene therapy products………………………………………………..5 3.4 General policies for glycosylated and non-glycosylated compounds………...6 3.5 General policies for immunoglobulins……………………………………………………….7 3.6 General polices for monoclonal antibodies………………………………………………..7 3.7 General polices for skin substitutes……………………………………………………………9 3.8 General policies for transgenic products……………………………………………………9 -

A Novel Treatment of Patients with Chronic Hepatitis C
A Novel Treatment of Patients with Chronic Hepatitis C Naoky CS. Tsai MD, Neil Shimoda MD, Linda Wong MD, Stanley Shimoda MD, Kimberly Goad RN, Herbert Yee, Miles Chen Abstract: interferon dosage, iron depletion therapy, and prolonged interferon Objectives: Interferon alpha-2b therapy for Chronic Hepatitis C therapy, but the beneficial results have not yet been established. patients has been unsatisfactory. Recombinant Granulocyte Mac Among the different groups of biological response modifiers, rophage Colony-Stimulating Factor has been shown to have anti- GM-CSF (Granulocyte Macrophage-Colony Stimulating Factor) is l23 Recently its viral effects in vivo and in vitro via cytokines a hormone-like glycoprotein cytokine produced by activated T hepatitis B and possibly chronic hepatitis C were effects on chronic lymphocytes, endothelial cells and fibroblasts that stimulates the reported.4’5 We, decided to conduct a pilot study to evaluate the proliferation, maturation and function of hemopoietic cells, aug anti-viral effects of recombinant human GM-CSF mono-therapy in and regulates the secretion patients with chronic hepatitis C and to assess its side effects, ments and modifies the immune system, Methods: A total of 10 patients (male/female: 5/5) (age: 34-60, of other cytokines which are involved in the immune response to mean: 45) seen in our center between 2/95 to 2/96 were randomly viral hepatitis. Recently J. Martin. et al reported HBV-DNA level selected to receive recombThant human Granulocyte Macrophage reduction with GM-CSF alone or in combination with interferon Colony-Stimulating-Factor at 125 ug/m2 subcutaneously daily for alfa-2b.2 Furthermore, in vitro studies of cytokine production by by three times weekly for another 8 weeks. -

Drug Use Evaluation: Colony Stimulating Factor Use in Patients with Hepatitis C
Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Drug Use Evaluation: Colony Stimulating Factor use in patients with Hepatitis C BACKGROUND Colony stimulating factors (CSFs) have been suggested for the off-label treatment of neutropenia caused by peg-interferon alfa treatment for hepatitis C.[1] The use of CSFs for the treatment of neutropenia is thought to increase the sustained virologic response (SVR) by maintaining therapeutic levels of peg-interferon alfa. Although CSFs for the treatment of neutropenia seems promising, it is unlikely that the benefits of use for outweigh the risks. Patient who are treated with CSFs are at an increased risk of developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).[2] There is low level of evidence supporting CSFs superiority over temporarily reducing the peg-interferon alfa dose.[3] According the American Association for the Study of Liver Guidelines and Veterans Affairs (VA) guidelines CSFs should only be considered when the patient experiences persistent neutropenia despite dose reduction of peg-interferon alpha. Factors that put patients with hepatitis C at a higher risk for neutropenia and possibly a higher response rate to CSFs include HIV infection, liver cirrhosis, or a liver transplant.[4,5] Goal: To evaluate CSFs use in hepatitis C patient. If inappropriate use was found, further prior authorization criteria would be brought to the committee for review. -

Addressing Challenges in Access to Oncology Medicines
Addressing Challenges in Access to Oncology Medicines Analytical Report ADDRESSING CHALLENGES IN ACCESS TO ONCOLOGY MEDICINES Analytical Report 2 Acknowledgements This analytical report was prepared by the Health Division, OECD Directorate for Employment, Labour and Social Affairs. The OECD Health Division would like to acknowledge the valuable contributions from the OECD Expert Group on Pharmaceuticals and Medical Devices. Writing this report would not have been possible without the contribution of experts from the OECD and EU countries. The authors are also grateful to various members of the Expert Group for input to the research for this paper as well as review of prior drafts. This paper benefited from review and feedback by Ruth Lopert and Francesca Colombo at the OECD Directorate for Employment, Labour and Social Affairs. Prior versions of this paper were reviewed by colleagues at the European Commission. This document, as well as any data and map included herein, are without prejudice to the status of or sovereignty over any territory, to the delimitation of international frontiers and boundaries and to the name of any territory, city or area. The statistical data for Israel are supplied by and under the responsibility of the relevant Israeli authorities. The use of such data by the OECD is without prejudice to the status of the Golan Heights, East Jerusalem and Israeli settlements in the West Bank under the terms of international law. This document was produced with the financial assistance of the European Union under The Third Health Programme 2014-2020. The contents of this report are the sole responsibility of the OECD and can in no way be taken to reflect the views of the European Union. -

Pegasys, INN-Peginterferon Alfa-2A
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Pegasys 180 micrograms solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Pegasys 180 micrograms solution for injection Each vial of 1 ml solution contains 180 micrograms peginterferon alfa-2a*. The strength indicates the quantity of the interferon alfa-2a moiety of peginterferon alfa-2a without consideration of the pegylation. *The active substance, peginterferon alfa-2a, is a covalent conjugate of the protein interferon alfa-2a produced by recombinant DNA technology in Escherichia coli with bis-[monomethoxy polyethylene glycol]. The potency of this medicinal product should not be compared to the one of another pegylated or non- pegylated protein of the same therapeutic class. For more information, see section 5.1. Excipient with known effect: Benzyl alcohol (10 mg/ 1 ml) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection (injection). The solution is clear and colourless to light yellow. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Chronic hepatitis B Adult patients Pegasys is indicated for the treatment of hepatitis B envelope antigen (HBeAg)-positive or HBeAg- negative chronic hepatitis B (CHB) in adult patients with compensated liver disease and evidence of viral replication, increased alanine aminotransferase (ALT) and histologically verified liver inflammation and/or fibrosis (see sections 4.4 and 5.1). Paediatric patients 3 years of age and older Pegasys is indicated for the treatment of HBeAg-positive CHB in non-cirrhotic children and adolescents 3 years of age and older with evidence of viral replication and persistently elevated serum ALT levels.