Direct Effects of Type I Interferons on Cells of the Immune System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lenograstim in the Treatment of Severe Neutropenia in Patients Treated with Peg-IFN and Ribavirin
Le Infezioni in Medicina, n. 1, 21-23, 2009 Lavori originali Lenograstim in the treatment of severe neutropenia in Original articles patients treated with Peg-IFN and ribavirin: the experience of a single hepatology unit Trattamento mediante lenograstim della neutropenia grave in corso di terapia con Peg-IFN e ribavirina: esperienza di una singola unità di epatologia Luciano Tarantino1, Annunziata De Rosa2, Orsola Tambaro1, Carmine Ripa1, Marta Celiento3, Antonio Schiano1 1Unità di Epatologia ed Ecointerventistica, Ospedale San Giovanni di Dio, Frattamaggiore, Napoli, Italy; 2AOU “Federico II”, Dipartimento di Malattie Infettive, ASL NA 2, Napoli, Italy; 3AOU “Federico II”, Dipartimento di Chirurgia Generale e Geriatria, ASL NA 2, Napoli, Italy n INTRODUCTION doses of 80 mcg s.c./week of Peg-IFN 2b and 1200 mg/die of ribavirin. We checked quanti- he standard treatment of chronic hepatitis C tative HCV- RNA at 12 and 48 weeks of treat- (ECA C) is pegylated interferon-alpha (Peg- ment. Every two weeks, two days before ad- TIFN ) combined with ribavirin [1]. One of the ministration of the weekly dose of Peg-IFN 2b most frequent causes of suspension of therapy or all patients had blood count and liver enzyme reduction of the doses of Peg-IFN is haemato- tests. Patients with absolute neutrophil counts toxicity, which includes anaemia and/or neu- <900 cells/mmc started on lenograstim (263 tropenia and/or thrombocytopenia [2]. The use µg) 24 hours before administration of Peg- of growth factors (G-CSF) in responder patients IFNα 2b (Figure 1). Indication for administer- allows effective doses of Peg-IFN and RIBA to be ing lenograstim was early viral response kept stable [3]. -

Type I Interferons in Anticancer Immunity
REVIEWS Type I interferons in anticancer immunity Laurence Zitvogel1–4*, Lorenzo Galluzzi1,5–8*, Oliver Kepp5–9, Mark J. Smyth10,11 and Guido Kroemer5–9,12 Abstract | Type I interferons (IFNs) are known for their key role in antiviral immune responses. In this Review, we discuss accumulating evidence indicating that type I IFNs produced by malignant cells or tumour-infiltrating dendritic cells also control the autocrine or paracrine circuits that underlie cancer immunosurveillance. Many conventional chemotherapeutics, targeted anticancer agents, immunological adjuvants and oncolytic 1Gustave Roussy Cancer Campus, F-94800 Villejuif, viruses are only fully efficient in the presence of intact type I IFN signalling. Moreover, the France. intratumoural expression levels of type I IFNs or of IFN-stimulated genes correlate with 2INSERM, U1015, F-94800 Villejuif, France. favourable disease outcome in several cohorts of patients with cancer. Finally, new 3Université Paris Sud/Paris XI, anticancer immunotherapies are being developed that are based on recombinant type I IFNs, Faculté de Médecine, F-94270 Le Kremlin Bicêtre, France. type I IFN-encoding vectors and type I IFN-expressing cells. 4Center of Clinical Investigations in Biotherapies of Cancer (CICBT) 507, F-94800 Villejuif, France. Type I interferons (IFNs) were first discovered more than Type I IFNs in cancer immunosurveillance 5Equipe 11 labellisée par la half a century ago as the factors underlying viral inter Type I IFNs are known to mediate antineoplastic effects Ligue Nationale contre le ference — that is, the ability of a primary viral infection against several malignancies, which is a clinically rel Cancer, Centre de Recherche 1 des Cordeliers, F-75006 Paris, to render cells resistant to a second distinct virus . -

Pegasys, INN-Peginterferon Alfa-2A
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion and scientific discussion on procedures, which have been finalised before 1 April 2005. For scientific information on procedures after this date please refer to module 8B. 1. Introduction Peginterferon alfa-2a is a polyethylene glycol (PEG)-modified form of human recombinant interferon alfa-2a intended for the treatment of adult patients with chronic hepatitis C (CHC) or chronic hepatitis B (CHB). Chronic hepatitis C is a major public health problem: hepatitis C virus (HCV) is responsible for a large proportion of chronic liver disease, accounting for 70% of cases of chronic hepatitis in industrialised countries. Globally there are an estimated 150 million chronic carriers of the virus, including 5 million in Western Europe. Without treatment approximately 30% of those infected with HCV will develop cirrhosis over a time frame of 30 years or more. For those with HCV-related cirrhosis, the prognosis is poor – a significant proportion will develop a life-threatening complication (either decompensated liver disease or an hepatocellular carcinoma) within a few years. The only therapy for those with advanced cirrhosis is liver transplantation, which carries a high mortality. In those who survive transplantation, viral recurrence in the new liver is almost inevitable and a significant proportion of infected liver grafts develop a progressive fibrosis that leads to recurrence of cirrhosis within 5 years. Interferon alfa monotherapy has been shown to be effective for the treatment of chronic hepatitis although sustained response rates occurred in approximately 15 to 30 % of patients treated for long duration (12-18 months). The current reference therapy is interferon alpha in combination with ribavirin, which resulted in an increase in biochemical and virological sustained response rates to approximately 40 % in naïve patients. -

Alfa Interferons
Clinical Pharmacy Program Guidelines for Alfa Interferons Program Prior Authorization Medication Intron A (interferon alfa-2b), Pegasys (peginterferon alfa-2a), PegIntron and Sylatron™ (peginterferon alfa-2b) Markets in Scope California,Colorado,Hawaii, Maryland, Nevada, New Jersey, New York, New York EPP, Pennsylvania- CHIP, Rhode Island, South Carolina Issue Date 9/2009 Pharmacy and 11/2020 Therapeutics Approval Date Effective Date 12/2020 1. Background: Indications Intron A (interferon alfa-2b) is indicated for the treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease who have a history of blood or blood- product exposure and/or are HCV antibody positive. Intron A has additional FDA labeling for the treatment of chronic hepatitis C in patients 3 years of age and older with compensated liver disease previously untreated with alpha interferon therapy and in patients 18 years of age and older who have relapsed following alpha interferon therapy. Intron A is also indicated for the treatment of chronic hepatitis B in patients 1 year of age or older with compensated liver disease. Patients who have been serum HBsAg positive for at least 6 months and have evidence of HBV replication (serum HBeAg positive) with elevated serum ALT are candidates for treatment. Intron A is indicated for the treatment of patients 18 years of age or older with hairy cell leukemia. Intron A is indicated as adjuvant to surgical treatment in patients 18 years of age or older with malignant melanoma who are free of disease but a high risk for systemic recurrence, within 56 days of surgery. -

Detecting the Stable Point of Therapeutic Effect of Chronic Myeloid
Xu et al. BMC Bioinformatics 2019, 20(Suppl 7):202 https://doi.org/10.1186/s12859-019-2738-0 RESEARCH Open Access Detecting the stable point of therapeutic effect of chronic myeloid leukemia based on dynamic network biomarkers Junhua Xu1,MinWu1, Shanshan Zhu1,JinzhiLei2 and Jie Gao1* From The 12th International Conference on Computational Systems Biology (ISB 2018) Guiyang, China. 18-21 August 2018 Abstract Background: Most researches of chronic myeloid leukemia (CML) are currently focused on the treatment methods, while there are relatively few researches on the progress of patients’ condition after drug treatment. Traditional biomarkers of disease can only distinguish normal state from disease state, and cannot recognize the pre-stable state after drug treatment. Results: A therapeutic effect recognition strategy based on dynamic network biomarkers (DNB) is provided for CML patients’ gene expression data. With the DNB criteria, the DNB with 250 genes is selected and the therapeutic effect index (TEI) is constructed for the detection of individual disease. The pre-stable state before the disease condition becomes stable is 1 month. Through functional analysis for the DNB, some genes are confirmed as key genes to affect the progress of CML patients’ condition. Conclusions: The results provide a certain theoretical direction and theoretical basis for medical personnel in the treatment of CML patients, and find new therapeutic targets in the future. The biomarkers of CML can help patients to be treated promptly and minimize drug resistance, treatment failure and relapse, which reduce the mortality of CML significantly. Keywords: Chronic myeloid leukemia (CML), Dynamic network biomarkers (DNB), Differentially expressed genes (DEGs), Therapeutic effect index (TEI), Pre-stable state, Treatment time Introduction At present, the use of ABL kinase inhibitors (e.g. -

Mechanisms of Type-I Ifn Inhibition: Equine Herpesvirus-1 Escape from the Antiviral Effect of Type-1 Interferon Response in Host Cell
University of Kentucky UKnowledge Theses and Dissertations--Veterinary Science Veterinary Science 2019 MECHANISMS OF TYPE-I IFN INHIBITION: EQUINE HERPESVIRUS-1 ESCAPE FROM THE ANTIVIRAL EFFECT OF TYPE-1 INTERFERON RESPONSE IN HOST CELL Fatai S. Oladunni University of Kentucky, [email protected] Author ORCID Identifier: https://orcid.org/0000-0001-5050-0183 Digital Object Identifier: https://doi.org/10.13023/etd.2019.374 Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Oladunni, Fatai S., "MECHANISMS OF TYPE-I IFN INHIBITION: EQUINE HERPESVIRUS-1 ESCAPE FROM THE ANTIVIRAL EFFECT OF TYPE-1 INTERFERON RESPONSE IN HOST CELL" (2019). Theses and Dissertations--Veterinary Science. 43. https://uknowledge.uky.edu/gluck_etds/43 This Doctoral Dissertation is brought to you for free and open access by the Veterinary Science at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Veterinary Science by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -
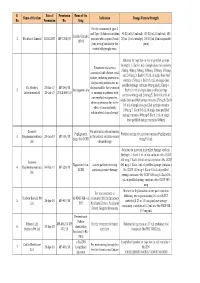
Final List of R-DNA Based Drugs Approved in the Country.Xlsx
S. Date of Permission Name of the Name of the firm Indication Dosage Form & Strength No. Permission No. Drug For the treatment of type -I and Type -II diabetes mellitus 40 IU/ml (10 ml vial), 100 IU/ml (10 ml vial), 100 Insulin Glargine 1 Wockhardt Limited 22.02.2007 MF-7206/07 patients who required basal IU/ml (3 ml cartridge), 100 IU/ml (3 ml disposable 100 IU (long acting) insulin for the pens) control of hyperglycemia. Solution for injection in vial or prefilled syringe Strength: 1. Each 1 mL of single dose vial contains Treatment of anaemia 25mcg, 40mcg, 60mcg, 100mcg, 200mcg, 300mcg associated with chronic renal and 500mcg 2. Each 0.75 mL of single dose vial failure, including patients on contains 150mcg 3. Each 0.3 mL of single dose dialysis and patients not on prefilled syringe contains 60mcg and 150mcg 4. Dr. Reddy's 23-Mar-10 MF-246/10 dialysis and for the treatment 2 Darbepoetin alfa Each 0.4 mL of single dose prefilled syringe Laboratories Ltd. 20-Jul-10 BULK-668/10 of anaemia in patients with contains 40mcg and 200mcg 5. Each 0.42 mL of non-myeloid malignancies, single dose prefilled syringe contains 25mcg 6. Each where anaemia is due to the 0.5 mL of single dose prefilled syringe contains effect of concomitantly 100mcg 7. Each 0.6 mL of single dose prefilled administered chemotherapy syringe contains 300mcg 8. Each 1 mL of single dose prefilled syringe contains 500mcg Gennova For prevention of neutropenia Pegfilgrastim Prefilled syringe for injection contains Pegfilgrastim 3 Biopharmaceuticals 29-Jan-10 MF-104/10 in the patient receiving cancer (peg-r-hu-GCSF) 6 mg/0.6 mL Ltd. -

Interferon-Alpha-Based Immunotherapies in the Treatment Of
Zhang et al. Exp Hematol Oncol (2017) 6:20 DOI 10.1186/s40164-017-0081-6 Experimental Hematology & Oncology REVIEW Open Access Interferon‑alpha‑based immunotherapies in the treatment of B cell‑derived hematologic neoplasms in today’s treat‑to‑target era Li Zhang1,2, Yu‑Tzu Tai1*, Matthew Zhi Guang Ho1,3,4, Lugui Qiu5 and Kenneth C. Anderson1* Abstract B cell lymphoma and multiple myeloma (MM) are the most common hematological malignancies which beneft from therapeutic monoclonal antibodies (mAbs)-based immunotherapies. Despite signifcant improvement on patient outcome following the use of novel therapies for the past decades, curative treatment is unavailable for the major‑ ity of patients. For example, the 5-year survival of MM is currently less than 50%. In the 1980s, interferon-α was used as monotherapy in newly diagnosed or previously treated MM with an overall response rate of 15–20%. Noticeably, a small subset of patients who responded to long-term interferon-α further achieved sustained complete remission. Since 1990, interferon-α-containing regimens have been used as a central maintenance strategy for patients with MM. However, the systemic administration of interferon-α was ultimately limited by its pronounced toxicity. To address this, the selective mAb-mediated delivery of interferon-α has been developed to enhance specifc killing of MM and B-cell malignant cells. As such, targeted interferon-α therapy may improve therapeutic window and sustain responses, while further overcoming suppressive microenvironment. This review aims to reinforce the role of interferon-α by consolidating our current understanding of targeting interferon-α with tumor-specifc mAbs for B cell lymphoma and myeloma. -

Wright1260921490.Pdf (1.84
A POTENTIAL STRATEGY TO MAINTAIN HSV-1 IN A LATENT STATE: USE OF IMMUNOREGULATORY PEPTIDE MIMETICS A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By NASRIN MAJIDI 2009 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES December 7, 2009 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Nasrin Majidi ENTITLED. A potential strategy to maintain HSV-1 in a latent state: use of immunoregulatory peptide mimetics. BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF Master of Science. ___________________________ Nancy J. Bigley, Ph.D. Thesis Director ___________________________ Barbara E. Hull, Ph.D. Program Director Committee on Final Examination _______________________ Nancy J. Bigley, Ph.D. _______________________ Barbara E. Hull, Ph.D. _______________________ Cheryl L. Conley, Ph.D. _______________________ Dean Joseph F. Thomas, Jr., Ph.D. Dean, School of Graduate Studies ABSTRACT Majidi, Nasrin. M.S., Department of Biological Sciences, Microbiology and Immunology Program, Wright State University, 2009. A potential strategy to maintain HSV-1 in a latent state: use of immunoregulatory peptide mimetics. This study reviews the role of interferon-gamma (IFN-γ) in HSV-1 latency. CD8+ T cells inhibit the reactivation of HSV-1 in trigeminal ganglia (TG) by production of IFN-γ. Although CD8+ T cells include all the cytotoxic apparatus for cytotoxicity, latently infected neuronal cells are not killed by CD8+ T cells. The CD94-NKG2a molecule on CD8+ T cells, binds to Qa-1b (a MHC class I like molecule) present on neuronal cell to inhibit CD8+ T cells cytotoxicity. -

Β Primary Human Leukocyte Subsets to IFN- Major Differences in The
Major Differences in the Responses of Primary Human Leukocyte Subsets to IFN- β Anette H. H. van Boxel-Dezaire, Joana A. Zula, Yaomin Xu, Richard M. Ransohoff, James W. Jacobberger and George R. This information is current as Stark of October 1, 2021. J Immunol published online 18 October 2010 http://www.jimmunol.org/content/early/2010/10/18/jimmun ol.0902314 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2010/10/19/jimmunol.090231 Material 4.DC1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on October 1, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published October 18, 2010, doi:10.4049/jimmunol.0902314 The Journal of Immunology Major Differences in the Responses of Primary Human Leukocyte Subsets to IFN-b Anette H. H. van Boxel-Dezaire,* Joana A. Zula,* Yaomin Xu,† Richard M. Ransohoff,‡ James W. Jacobberger,x and George R. Stark* Treatment of cell lines with type I IFNs activates the formation of IFN-stimulated gene factor 3 (STAT1/STAT2/IFN regulatory factor-9), which induces the expression of many genes. -

Interferon-Alpha Promotes Immunosuppression Through IFNAR1/STAT1 Signalling in Head and Neck Squamous Cell Carcinoma
www.nature.com/bjc ARTICLE Translational Therapeutics Interferon-alpha promotes immunosuppression through IFNAR1/STAT1 signalling in head and neck squamous cell carcinoma Hailong Ma1,2, Wenyi Yang1,2, Liming Zhang1,2, Shuli Liu1,2, Mei Zhao3, Ge Zhou3, Lizhen Wang4, Shufang Jin1,2, Zhiyuan Zhang1,2 and Jingzhou Hu1,2 BACKGROUND: An immunosuppressive microenvironment is critical for cancer initiation and progression. Whether interferon alpha (IFNα) can suppress immune and cancer cells and its involved mechanism still remain largely elusive. METHODS: We examine the expression of interferon alpha/beta receptor-1 (IFNAR1), CD8, CD56 and programmed death ligand 1 (PDL1) in head and neck squamous cell carcinomas (HNSCC). The effect of IFNα on PDL1 and programmed cell death protein 1 (PD1) expression in tumour cells and immune cells was detected in vitro and in vivo. RESULTS: Overexpression of IFNAR1, MX1 and signal transducer and activator of transcription 1 (Stat1) indicated the endogenous IFNα activation in tumour microenvironment, which correlated with immunosuppression status in HNSCC patients. Moreover, IFNα transcriptionally activated the expression of PDL1 through p-Stat1 (Tyr701) and promoted PD1 expression in immune cells through IFNAR1. The inhibition of IFNα signalling enhanced the cytotoxic activity of nature killer cells. At lastastly, we confirmed the upregulation of PDL1 and PD1 in response to IFNα treatment in both xenograft tumour models and patient-derived xenograft models. CONCLUSIONS: Our findings demonstrate that IFNα-induced PDL1 and PD1 expression is a new mechanism of immunosuppression in HNSCC, suggesting that blocking IFNα signalling may enhance the efficacy of immune checkpoint blockade. British Journal of Cancer (2019) 120:317–330; https://doi.org/10.1038/s41416-018-0352-y INTRODUCTION Interferon alpha (IFNα) is a pleiotropic cytokine belonging to the Head and neck squamous cell carcinoma (HNSCC) accounts for ~ type I IFN family that is originally described for its antiviral 90% of head and neck cancer. -

Innate and Adaptive Signals Enhance Differentiation and Expansion of Dual-Antibody Autoreactive B Cells in Lupus
ARTICLE DOI: 10.1038/s41467-018-06293-z OPEN Innate and adaptive signals enhance differentiation and expansion of dual-antibody autoreactive B cells in lupus Allison Sang1, Thomas Danhorn 2, Jacob N. Peterson1, Andrew L. Rankin3,4, Brian P. O’Connor1,2,5,6, Sonia M. Leach2,5, Raul M. Torres1,5 & Roberta Pelanda1,5 1234567890():,; Autoreactive B cells have a major function in autoimmunity. A small subset of B cells expressing two distinct B-cell-antigen-receptors (B2R cells) is elevated in many patients with systematic lupus erythematosus (SLE) and in the MRL(/lpr) mouse model of lupus, and is often autoreactive. Here we show, using RNAseq and in vitro and in vivo analyses, signals that are required for promoting B2R cell numbers and effector function in autoimmune mice. Compared with conventional B cells, B2R cells are more responsive to Toll-like receptor 7/9 and type I/II interferon treatment, display higher levels of MHCII and co-receptors, and depend on IL-21 for their homeostasis; moreover they expand better upon T cell-dependent antigen stimulation, and mount a more robust memory response, which are characteristics essential for enhanced (auto)immune responses. Our findings thus provide insights on the stimuli for the expansion of an autoreactive B cell subset that may contribute to the etiology of SLE. 1 Department of Immunology and Microbiology, University of Colorado School of Medicine, Aurora, CO 80045, USA. 2 Center for Genes, Environment and Health, National Jewish Health, Denver, CO 80206, USA. 3 Inflammation and Immunology, Pfizer Research, Cambridge, MA 02140, USA. 4 Immuno- Oncology Discovery, FivePrime Therapeutics, South San Francisco, CA 94080, USA.