Withdrawal of the Marketing Authorisation Application for Joulferon (Albinterferon Alfa-2B)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lenograstim in the Treatment of Severe Neutropenia in Patients Treated with Peg-IFN and Ribavirin
Le Infezioni in Medicina, n. 1, 21-23, 2009 Lavori originali Lenograstim in the treatment of severe neutropenia in Original articles patients treated with Peg-IFN and ribavirin: the experience of a single hepatology unit Trattamento mediante lenograstim della neutropenia grave in corso di terapia con Peg-IFN e ribavirina: esperienza di una singola unità di epatologia Luciano Tarantino1, Annunziata De Rosa2, Orsola Tambaro1, Carmine Ripa1, Marta Celiento3, Antonio Schiano1 1Unità di Epatologia ed Ecointerventistica, Ospedale San Giovanni di Dio, Frattamaggiore, Napoli, Italy; 2AOU “Federico II”, Dipartimento di Malattie Infettive, ASL NA 2, Napoli, Italy; 3AOU “Federico II”, Dipartimento di Chirurgia Generale e Geriatria, ASL NA 2, Napoli, Italy n INTRODUCTION doses of 80 mcg s.c./week of Peg-IFN 2b and 1200 mg/die of ribavirin. We checked quanti- he standard treatment of chronic hepatitis C tative HCV- RNA at 12 and 48 weeks of treat- (ECA C) is pegylated interferon-alpha (Peg- ment. Every two weeks, two days before ad- TIFN ) combined with ribavirin [1]. One of the ministration of the weekly dose of Peg-IFN 2b most frequent causes of suspension of therapy or all patients had blood count and liver enzyme reduction of the doses of Peg-IFN is haemato- tests. Patients with absolute neutrophil counts toxicity, which includes anaemia and/or neu- <900 cells/mmc started on lenograstim (263 tropenia and/or thrombocytopenia [2]. The use µg) 24 hours before administration of Peg- of growth factors (G-CSF) in responder patients IFNα 2b (Figure 1). Indication for administer- allows effective doses of Peg-IFN and RIBA to be ing lenograstim was early viral response kept stable [3]. -

Current Therapies for Chronic Hepatitis C
Southern Illinois University Edwardsville SPARK Pharmacy Faculty Research, Scholarship, and Creative Activity School of Pharmacy 1-2011 Current Therapies for Chronic Hepatitis C McKenzie C. Ferguson Southern Illinois University Edwardsville, [email protected] Follow this and additional works at: https://spark.siue.edu/pharmacy_fac Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Ferguson, McKenzie C., "Current Therapies for Chronic Hepatitis C" (2011). Pharmacy Faculty Research, Scholarship, and Creative Activity. 4. https://spark.siue.edu/pharmacy_fac/4 This Article is brought to you for free and open access by the School of Pharmacy at SPARK. It has been accepted for inclusion in Pharmacy Faculty Research, Scholarship, and Creative Activity by an authorized administrator of SPARK. For more information, please contact [email protected],[email protected]. Chronic Hepatitis C & Current Therapies 1 Review of Chronic Hepatitis C & Current Therapies Reviews of Therapeutics Pharmacotherapy McKenzie C. Ferguson, Pharm.D., BCPS From the School of Pharmacy, Southern Illinois University Edwardsville, Department of Pharmacy Practice, Edwardsville, Illinois. Address for reprint requests : Southern Illinois University Edwardsville School of Pharmacy 220 University Park Drive, Ste 1037 Edwardsville, IL 62026-2000 Email: [email protected] Keywords : hepatitis C, HCV, ribavirin, interferon, peginterferon alfa-2a, peginterferon alfa-2b, albinterferon, taribavirin, telaprevir, therapeutic efficacy, safety The author received no sources of support in the form of grants, equipment, or drugs. Chronic Hepatitis C & Current Therapies 2 ABSTRACT Hepatitis C virus affects more than 180 million people worldwide and as many as 4 million people in the United States. Given that most patients are asymptomatic until late in disease progression, diagnostic screening and evaluation of patients that display high-risk behaviors associated with acquisition of hepatitis C should be performed. -

Pegasys, INN-Peginterferon Alfa-2A
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion and scientific discussion on procedures, which have been finalised before 1 April 2005. For scientific information on procedures after this date please refer to module 8B. 1. Introduction Peginterferon alfa-2a is a polyethylene glycol (PEG)-modified form of human recombinant interferon alfa-2a intended for the treatment of adult patients with chronic hepatitis C (CHC) or chronic hepatitis B (CHB). Chronic hepatitis C is a major public health problem: hepatitis C virus (HCV) is responsible for a large proportion of chronic liver disease, accounting for 70% of cases of chronic hepatitis in industrialised countries. Globally there are an estimated 150 million chronic carriers of the virus, including 5 million in Western Europe. Without treatment approximately 30% of those infected with HCV will develop cirrhosis over a time frame of 30 years or more. For those with HCV-related cirrhosis, the prognosis is poor – a significant proportion will develop a life-threatening complication (either decompensated liver disease or an hepatocellular carcinoma) within a few years. The only therapy for those with advanced cirrhosis is liver transplantation, which carries a high mortality. In those who survive transplantation, viral recurrence in the new liver is almost inevitable and a significant proportion of infected liver grafts develop a progressive fibrosis that leads to recurrence of cirrhosis within 5 years. Interferon alfa monotherapy has been shown to be effective for the treatment of chronic hepatitis although sustained response rates occurred in approximately 15 to 30 % of patients treated for long duration (12-18 months). The current reference therapy is interferon alpha in combination with ribavirin, which resulted in an increase in biochemical and virological sustained response rates to approximately 40 % in naïve patients. -

Alfa Interferons
Clinical Pharmacy Program Guidelines for Alfa Interferons Program Prior Authorization Medication Intron A (interferon alfa-2b), Pegasys (peginterferon alfa-2a), PegIntron and Sylatron™ (peginterferon alfa-2b) Markets in Scope California,Colorado,Hawaii, Maryland, Nevada, New Jersey, New York, New York EPP, Pennsylvania- CHIP, Rhode Island, South Carolina Issue Date 9/2009 Pharmacy and 11/2020 Therapeutics Approval Date Effective Date 12/2020 1. Background: Indications Intron A (interferon alfa-2b) is indicated for the treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease who have a history of blood or blood- product exposure and/or are HCV antibody positive. Intron A has additional FDA labeling for the treatment of chronic hepatitis C in patients 3 years of age and older with compensated liver disease previously untreated with alpha interferon therapy and in patients 18 years of age and older who have relapsed following alpha interferon therapy. Intron A is also indicated for the treatment of chronic hepatitis B in patients 1 year of age or older with compensated liver disease. Patients who have been serum HBsAg positive for at least 6 months and have evidence of HBV replication (serum HBeAg positive) with elevated serum ALT are candidates for treatment. Intron A is indicated for the treatment of patients 18 years of age or older with hairy cell leukemia. Intron A is indicated as adjuvant to surgical treatment in patients 18 years of age or older with malignant melanoma who are free of disease but a high risk for systemic recurrence, within 56 days of surgery. -

Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation
Review Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation Leonor S. Castro 1,†, Guilherme S. Lobo 1,†, Patrícia Pereira 2 , Mara G. Freire 1 ,Márcia C. Neves 1,* and Augusto Q. Pedro 1,* 1 CICECO–Aveiro Institute of Materials, Chemistry Department, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal; [email protected] (L.S.C.); [email protected] (G.S.L.); [email protected] (M.G.F.) 2 Centre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra, Rua Sílvio Lima-Polo II, 3030-790 Coimbra, Portugal; [email protected] * Correspondence: [email protected] (M.C.N.); [email protected] (A.Q.P.) † These authors contributed equally to this work. Abstract: The advent of biopharmaceuticals in modern medicine brought enormous benefits to the treatment of numerous human diseases and improved the well-being of many people worldwide. First introduced in the market in the early 1980s, the number of approved biopharmaceutical products has been steadily increasing, with therapeutic proteins, antibodies, and their derivatives accounting for most of the generated revenues. The success of pharmaceutical biotechnology is closely linked with remarkable developments in DNA recombinant technology, which has enabled the production of proteins with high specificity. Among promising biopharmaceuticals are interferons, first described by Isaacs and Lindenmann in 1957 and approved for clinical use in humans nearly thirty years later. Interferons are secreted autocrine and paracrine proteins, which by regulating several biochemical Citation: Castro, L.S.; Lobo, G.S.; pathways have a spectrum of clinical effectiveness against viral infections, malignant diseases, Pereira, P.; Freire, M.G.; Neves, M.C.; and multiple sclerosis. -
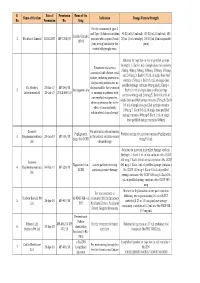
Final List of R-DNA Based Drugs Approved in the Country.Xlsx
S. Date of Permission Name of the Name of the firm Indication Dosage Form & Strength No. Permission No. Drug For the treatment of type -I and Type -II diabetes mellitus 40 IU/ml (10 ml vial), 100 IU/ml (10 ml vial), 100 Insulin Glargine 1 Wockhardt Limited 22.02.2007 MF-7206/07 patients who required basal IU/ml (3 ml cartridge), 100 IU/ml (3 ml disposable 100 IU (long acting) insulin for the pens) control of hyperglycemia. Solution for injection in vial or prefilled syringe Strength: 1. Each 1 mL of single dose vial contains Treatment of anaemia 25mcg, 40mcg, 60mcg, 100mcg, 200mcg, 300mcg associated with chronic renal and 500mcg 2. Each 0.75 mL of single dose vial failure, including patients on contains 150mcg 3. Each 0.3 mL of single dose dialysis and patients not on prefilled syringe contains 60mcg and 150mcg 4. Dr. Reddy's 23-Mar-10 MF-246/10 dialysis and for the treatment 2 Darbepoetin alfa Each 0.4 mL of single dose prefilled syringe Laboratories Ltd. 20-Jul-10 BULK-668/10 of anaemia in patients with contains 40mcg and 200mcg 5. Each 0.42 mL of non-myeloid malignancies, single dose prefilled syringe contains 25mcg 6. Each where anaemia is due to the 0.5 mL of single dose prefilled syringe contains effect of concomitantly 100mcg 7. Each 0.6 mL of single dose prefilled administered chemotherapy syringe contains 300mcg 8. Each 1 mL of single dose prefilled syringe contains 500mcg Gennova For prevention of neutropenia Pegfilgrastim Prefilled syringe for injection contains Pegfilgrastim 3 Biopharmaceuticals 29-Jan-10 MF-104/10 in the patient receiving cancer (peg-r-hu-GCSF) 6 mg/0.6 mL Ltd. -

7 Engineering of Therapeutic Proteins
Engineering of 7 Therapeutic Proteins Fei Wen, Sheryl B. Rubin-Pitel, and Huimin Zhao CONTENTS Protein Therapeutics versus Small Molecule Drugs .............................................. 154 Sources of Protein Therapeutics ................................................................... 155 Targets of Protein Therapeutics and Modes of Action .................................. 155 Engineering Effective Protein Therapeutics .......................................................... 156 Challenges in Pharmaceutical Translation of New Therapeutic Proteins ..... 156 Strategies for Designing Effective Protein Therapeutics .............................. 156 Strategies for Improving Pharmacokinetics ...................................... 157 Strategies for Reducing Immunogenicity ......................................... 158 Genetic Engineering ......................................................................... 159 Examples of Protein Therapeutics ......................................................................... 160 Monoclonal Antibody Therapeutics.............................................................. 161 Enzyme Therapeutics Acting on Extracellular Targets ................................. 161 Protein Therapeutics as Replacements for Defective or Deficient Proteins .....162 Protein Hormones ............................................................................. 162 Coagulation Factors .......................................................................... 163 Enzyme Replacement Therapy ........................................................ -

Part One General Information
1 Part One General Information 3 1 Half - Life Modulating Strategies – An Introduction Roland E. Kontermann 1.1 Therapeutic Proteins With roughly 200 biologics approved for therapeutic applications and more than 600 under clinical development [1] , biotechnology products cover an increased proportion of all therapeutic drugs. Besides monoclonal antibodies and vaccines, which account for more than two - thirds of these produces, hormones, growth factors, cytokines, fusion proteins, coagulation factors, enzymes and other pro- teins are listed. An overview of the different classes of currently approved protein therapeutics is shown in Table 1.1 . Except for antibodies and Fc fusion proteins, many of these proteins possess a molecular mass below 50 kDa and a rather short terminal half - life in the range of minutes to hours. In order to maintain a thera- peutically effective concentration over a prolonged period of time, infusions or frequent administrations are performed, or the drug is applied loco - regional or subcutaneously utilizing a slow adsorption into the blood stream. These limita- tions of small size protein drugs has led to the development and implementation of half - life extension strategies to prolong circulation of these recombinant anti- bodies in the blood and thus improve administration and pharmacokinetic as well as pharmacodynamic properties. 1.2 Renal Clearance and F c R n - Mediated Recycling The effi cacy of protein therapeutics is strongly determined by their pharmacoki- netic properties, including their plasma half - lives, which infl uence distribution and excretion. Although a small size facilitates tissue penetration, these molecules are often rapidly cleared from circulation. Thus, they have to be administered as infusion or repeated intravenous ( i.v. -

Use of Interferon Alfa in the Treatment of Myeloproliferative Neoplasms: Perspectives and Review of the Literature
cancers Review Use of Interferon Alfa in the Treatment of Myeloproliferative Neoplasms: Perspectives and Review of the Literature Joan How 1,2,3 and Gabriela Hobbs 1,* 1 Department of Medical Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; [email protected] 2 Division of Hematology, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA 3 Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115, USA * Correspondence: [email protected]; Tel.: +1-617-724-1124 Received: 26 June 2020; Accepted: 10 July 2020; Published: 18 July 2020 Abstract: Interferon alfa was first used in the treatment of myeloproliferative neoplasms (MPNs) over 30 years ago. However, its initial use was hampered by its side effect profile and lack of official regulatory approval for MPN treatment. Recently, there has been renewed interest in the use of interferon in MPNs, given its potential disease-modifying effects, with associated molecular and histopathological responses. The development of pegylated formulations and, more recently, ropeginterferon alfa-2b has resulted in improved tolerability and further expansion of interferon’s use. We review the evolving clinical use of interferon in essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF). We discuss interferon’s place in MPN treatment in the context of the most recent clinical trial results evaluating interferon and its pegylated formulations, and its role in special populations such as young and pregnant MPN patients. Interferon has re-emerged as an important option in MPN patients, with future studies seeking to re-establish its place in the existing treatment algorithm for MPN, and potentially expanding its use for novel indications and combination therapies. -

Interferon in Inflammatory Diseases
Meeting report Lupus Sci Med: first published as 10.1136/lupus-2018-000276 on 15 June 2018. Downloaded from Report of the inaugural Interferon Research Summit: interferon in inflammatory diseases Mary K Crow,1 Lars Rönnblom2 To cite: Crow MK, ABSTRACT responses to distinct classes of therapeutic Rönnblom L. Report of An international summit agents. the inaugural Interferon on interferon (IFN) in inflammatory diseases, held in The inaugural International Summit on Research Summit: interferon Gaithersburg, Maryland, USA (4–5 May 2017), united 22 in inflammatory diseases. IFN in Inflammatory Diseases united 22 inter- internationally renowned clinicians and scientists with Lupus Science & Medicine nationally renowned clinicians and scientists backgrounds in basic science, translational science and 2018;5:e000276. doi:10.1136/ with backgrounds in basic science, transla- lupus-2018-000276 clinical medicine. The objectives of the summit were to assess the current knowledge of the role of type I IFN in tional science and clinical medicine. Goals inflammatory diseases and other conditions, discuss the of the summit included assessing the current ► Additional material is available clinical trial data of anti-IFN therapeutic agents knowledge of the role type I IFN plays in published online only. To view and identify key clinical and therapeutic knowledge gaps inflammatory diseases and other conditions, please visit the journal online (http:// dx. doi. org/ 10. 1136/ 10. and future directions to advance the treatment landscape discussing available clinical data examining 1136/ lupus- 2018- 000276) of diseases involving the type I IFN pathway. A discussion- anti-IFN therapies and identifying current key based consensus process was used to assess three main clinical and therapeutic knowledge gaps. -

INTRON A, Interferon Alfa-2B
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT IntronA 3 million IU/0.5 mL solution for injection or infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial of solution for injection or infusion contains 3 million IU of recombinant interferon alfa-2b produced in E. coli by recombinant DNA technology, in 0.5 mL of solution. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection or infusion. Clear and colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Chronic hepatitis B Treatment of adult patients with chronic hepatitis B associated with evidence of hepatitis B viral replication (presence of DNA of hepatitis B virus (HBV-DNA) and hepatitis B antigen (HBeAg), elevated alanine aminotransferase (ALT) and histologically proven active liver inflammation and/or fibrosis. Chronic hepatitis C Before initiating treatment with IntronA, consideration should be given to the results from clinical trials comparing IntronA with pegylated interferon (see section 5.1). Adult patients IntronA is indicated for the treatment of adult patients with chronic hepatitis C who have elevated transaminases without liver decompensation and who are positive for hepatitis C virus RNA (HCV-RNA) (see section 4.4). The best way to use IntronA in this indication is in combination with ribavirin. Children 3 years of age and older and adolescents IntronA is indicated, in a combination regimen with ribavirin, for the treatment of children 3 years of age and older and adolescents, who have chronic hepatitis C, not previously treated, without liver decompensation, and who are positive for HCV-RNA. -

Diagnosis Code-Restricted Physician-Administered Drugs
Diagnosis Code-Restricted Physician-Administered Drugs The following table contains diagnosis-restricted physician-administered drugs and the corresponding diagnosis code and disease descriptions. When a physician-administered drug claim is submitted with a diagnosis listed in this attachment, prior authorization (PA) is not required. For uses outside the listed diagnosis, PA is required. Submission of peer-reviewed medical literature from scientific medical or pharmaceutical publications in which original manuscripts are rejected or published only after having been reviewed by unbiased independent experts to support the proven efficacy of the requested use of the drug is also required to be submitted with the PA request. Note: This table includes Wisconsin Medicaid’s most current information and may be updated periodically. Effective: July 1, 2012 HCPCSCode* Drug Name Diagnosis Code Disease Description J0205 Alglucerase (Ceredase) 2727 Gaucher’s Disease 3336 Idiopathic dystonia 3337 Symptomatic torsion dystonia 33381 Blepharospasm 33383 Spasmodic torticollis 33384 Focal hand dystonia Spastic hemiplegia and hemiparesis affecting 34211 dominant side Spastic hemiplegia and hemiparesis affecting 34212 Botulinum Toxin Type A nondominant side J0585 (Botox) 3440-34404, 34409 Quadriplegia 3441 Paraplegia 340 Multiple Sclerosis 3430-3439 Cerebral palsy 3518 Facial spasm 3780-37887 Strabismus 70521 Hyperhidrosis 72885 Spasm of muscle 7810 Hemifacial spasm Botulinum Toxin Type B J0587 33383 Spasmodic torticollis (Myobloc) J0740 Cidofovir (Vistide),