Peginterferon Alfa-2A, Pegasys
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Induction of a TRAIL Mediated Suicide Program by Interferon Alpha in Primary E€Usion Lymphoma
Oncogene (2001) 20, 7029 ± 7040 ã 2001 Nature Publishing Group All rights reserved 0950 ± 9232/01 $15.00 www.nature.com/onc Induction of a TRAIL mediated suicide program by interferon alpha in primary eusion lymphoma Ngoc L Toomey1,4, Vadim V Deyev2,4, Charles Wood3, Lawrence H Boise2, Duncan Scott1, Lei Hua Liu1, Lisa Cabral1, Eckhard R Podack2, Glen N Barber2 and William J Harrington Jr*,1 1Department of Medicine University of Miami School of Medicine, Miami, Florida, FL 33136, USA; 2Department of Microbiology and Immunology, University of Miami School of Medicine, Miami, Florida, FL 33136, USA; 3School of Biological Sciences, University of Nebraska, Lincoln, Nebraska, NE 68588, USA Gammaherpes viruses are often detected in lymphomas Virus (EBV) or Human Herpes Virus Type 8 (HHV-8) arising in immunocompromised patients. We have found have been isolated from lymphomas found in im- that Azidothymidine (AZT) alone induces apoptosis in munosuppressed organ transplant recipients, children Epstein Barr Virus (EBV) positive Burkitt's lymphoma with hereditary immunode®ciencies and patients with (BL) cells but requires interferon alpha (IFN-a) to induce acquired immunode®ciency (AIDS) (Swinnen, 1999; apoptosis in Human Herpes Virus Type 8 (HHV-8) Goldsby and Carroll, 1998; Knowles, 1999). Many of positive Primary Eusion Lymphomas (PEL). Our these tumors can be categorized into distinct subtypes analysis of a series of AIDS lymphomas revealed that based on a variety of morphologic and molecular IFN-a selectively induced very high levels of the Death criteria. For example, AIDS associated large cell Receptor (DR) tumor necrosis factor-related apoptosis- diuse or immunoblastic lymphomas (DLCL, IBL) inducing ligand (TRAIL) in HHV-8 positive PEL lines are often EBV positive while AIDS associated Burkitt's and primary tumor cells whereas little or no induction lymphomas (BL) less frequently contain EBV (Gaidano was observed in primary EBV+ AIDS lymphomas and et al., 1994). -

Lenograstim in the Treatment of Severe Neutropenia in Patients Treated with Peg-IFN and Ribavirin
Le Infezioni in Medicina, n. 1, 21-23, 2009 Lavori originali Lenograstim in the treatment of severe neutropenia in Original articles patients treated with Peg-IFN and ribavirin: the experience of a single hepatology unit Trattamento mediante lenograstim della neutropenia grave in corso di terapia con Peg-IFN e ribavirina: esperienza di una singola unità di epatologia Luciano Tarantino1, Annunziata De Rosa2, Orsola Tambaro1, Carmine Ripa1, Marta Celiento3, Antonio Schiano1 1Unità di Epatologia ed Ecointerventistica, Ospedale San Giovanni di Dio, Frattamaggiore, Napoli, Italy; 2AOU “Federico II”, Dipartimento di Malattie Infettive, ASL NA 2, Napoli, Italy; 3AOU “Federico II”, Dipartimento di Chirurgia Generale e Geriatria, ASL NA 2, Napoli, Italy n INTRODUCTION doses of 80 mcg s.c./week of Peg-IFN 2b and 1200 mg/die of ribavirin. We checked quanti- he standard treatment of chronic hepatitis C tative HCV- RNA at 12 and 48 weeks of treat- (ECA C) is pegylated interferon-alpha (Peg- ment. Every two weeks, two days before ad- TIFN ) combined with ribavirin [1]. One of the ministration of the weekly dose of Peg-IFN 2b most frequent causes of suspension of therapy or all patients had blood count and liver enzyme reduction of the doses of Peg-IFN is haemato- tests. Patients with absolute neutrophil counts toxicity, which includes anaemia and/or neu- <900 cells/mmc started on lenograstim (263 tropenia and/or thrombocytopenia [2]. The use µg) 24 hours before administration of Peg- of growth factors (G-CSF) in responder patients IFNα 2b (Figure 1). Indication for administer- allows effective doses of Peg-IFN and RIBA to be ing lenograstim was early viral response kept stable [3]. -

Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection Against Classical Swine Fever Virus
Article Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus Yusmel Sordo-Puga 1, Marisela Suárez-Pedroso 1 , Paula Naranjo-Valdéz 2, Danny Pérez-Pérez 1, Elaine Santana-Rodríguez 1, Talia Sardinas-Gonzalez 1, Mary Karla Mendez-Orta 1, Carlos A. Duarte-Cano 1, Mario Pablo Estrada-Garcia 1 and María Pilar Rodríguez-Moltó 1,* 1 Animal Biotechnology Department, Center for Genetic Engineering and Biotechnology, P.O. Box 6162, Havana 10600, Cuba; [email protected] (Y.S.-P.); [email protected] (M.S.-P.); [email protected] (D.P.-P.); [email protected] (E.S.-R.); [email protected] (T.S.-G.); [email protected] (M.K.M.O.); [email protected] (C.A.D.); [email protected] (M.P.E.) 2 Central Laboratory Unit for Animal Health (ULCSA), Havana 11400, Cuba; [email protected] * Correspondence: [email protected]; Tel.: +53-7-2504419 Abstract: Live attenuated C-strain classical swine fever vaccines provide early onset protection. These vaccines confer effective protection against the disease at 5–7 days post-vaccination. It was previously reported that intramuscular administration of the Porvac® vaccine protects against highly virulent Citation: Sordo-Puga, Y.; classical swine fever virus (CSFV) “Margarita” strain as early as seven days post-vaccination. In Suárez-Pedroso, M.; Naranjo-Valdéz, order to identify how rapidly protection against CSFV is conferred after a single dose of the Porvac® P.; Pérez-Pérez, D.; subunit vaccine E2-CD154, 15 swine, vaccinated with a single dose of Porvac®, were challenged Santana-Rodríguez, E.; 3 intranasally at five, three, and one day post-vaccination with 2 × 10 LD50 of the highly pathogenic Sardinas-Gonzalez, T.; Mendez-Orta, Cuban “Margarita” strain of the classical swine fever virus. -

Age- and Gender-Specific Modulation of Serum Osteopontin and Interferon-Α by Osteopontin Genotype in Systemic Lupus Er
Genes and Immunity (2009) 10, 487–494 & 2009 Macmillan Publishers Limited All rights reserved 1466-4879/09 $32.00 www.nature.com/gene ORIGINAL ARTICLE Age- and gender-specific modulation of serum osteopontin and interferon-a by osteopontin genotype in systemic lupus erythematosus SN Kariuki1, JG Moore1, KA Kirou2,MKCrow2, TO Utset1 and TB Niewold1 1Section of Rheumatology, University of Chicago, Chicago, IL, USA and 2Mary Kirkland Center for Lupus Research, Hospital for Special Surgery, New York, NY, USA Osteopontin (OPN) is a multifunctional cytokine involved in long bone remodeling and immune system signaling. Additionally, OPN is critical for interferon-a (IFN-a) production in murine plasmacytoid dendritic cells. We have previously shown that IFN-a is a heritable risk factor for systemic lupus erythematosus (SLE). Genetic variants of OPN have been associated with SLE susceptibility, and one study suggests that this association is particular to men. In this study, the 3 0 UTR SLE-risk variant of OPN (rs9138C) was associated with higher serum OPN and IFN-a in men (P ¼ 0.0062 and P ¼ 0.0087, respectively). In women, the association between rs9138 C and higher serum OPN and IFN-a was restricted to younger subjects, and risk allele carriers showed a strong age-related genetic effect of rs9138 genotype on both serum OPN and IFN-a (Po0.0001). In African- American subjects, the 5 0 region single nucleotide polymorphisms, rs11730582 and rs28357094, were associated with anti- RNP antibodies (odds ratio (OR) ¼ 2.9, P ¼ 0.0038 and OR ¼ 3.9, P ¼ 0.021, respectively). Thus, we demonstrate two distinct genetic influences of OPN on serum protein traits in SLE patients, which correspond to previously reported SLE-risk variants. -

Interferon-Γ Enhances Interleukin 12 Production in Rheumatoid Synovial
Interferon-γ Enhances Interleukin 12 Production in Rheumatoid Synovial Cells via CD40-CD154 Dependent and Independent Pathways MINETAKE KITAGAWA, HIROSHI SUZUKI, YOSHIHIRO ADACHI, HIROSHI NAKAMURA, SHINICHI YOSHINO, and TAKAYUKI SUMIDA ABSTRACT. Objective. To determine the role of interferon-γ (IFN-γ) in CD40-CD154 dependent production of interleukin 12 (IL-12) by synovial cells of patients with rheumatoid arthritis (RA). Methods. We examined the effects of IFN-γ, tumor necrosis factor-α (TNF-α), and granulocyte- macrophage colony stimulating factor (GM-CSF) on CD40 expression on CD68+ synovial macrophage-lineage cells (SMC). The effects of IFN-γ and soluble CD154 (sCD154) on IL-12 production by RA synovial cells were determined by ELISA. Results. CD68+ SMC expressed substantial levels of CD40. IFN-γ, but not TNF-α or GM-CSF, markedly upregulated CD40 expression on CD68+ SMC. IFN-γ also dose dependently increased IL- γ 12 production by synovial cells. The effects of IFN- on CD40 expression (EC50 = 127.4 U/ml) were observed at a concentration 19 times lower than the effects on IL-12 production (EC50 = 6.8 U/ml). Treatment with IFN-γ at a concentration low enough to augment CD40 expression but not IL-12 production enhanced spontaneous IL-12 production synergy with sCD154. The synergistic enhance- ment of spontaneous IL-12 production was abrogated by CD40-Fc. In contrast, IL-12 production induced by high concentration of IFN-γ was not neutralized by CD40-Fc. Conclusion. IFN-γ enhanced IL-12 production via both CD40-CD154 dependent and independent pathways in RA synovium. IFN-γ may play a crucial role in the development of RA synovitis through regulation of IL-12 production. -

Dysregulated Interferon Response Underlying Severe COVID-19
viruses Review Dysregulated Interferon Response Underlying Severe COVID-19 LeAnn Lopez y, Peter C. Sang y, Yun Tian y and Yongming Sang * Department of Agricultural and Environmental Sciences, College of Agriculture, Tennessee State University, 3500 John A. Merritt Boulevard, Nashville, TN 37209, USA; [email protected] (L.L.); [email protected] (P.C.S.); [email protected] (Y.T.) * Correspondence: [email protected]; Tel.: +1-615-963-5183 These authors contributed equally. y Academic Editor: Andrew Davidson Received: 27 October 2020; Accepted: 9 December 2020; Published: 13 December 2020 Abstract: Innate immune interferons (IFNs), including type I and III IFNs, constitute critical antiviral mechanisms. Recent studies reveal that IFN dysregulation is key to determine COVID-19 pathogenesis. Effective IFN stimulation or prophylactic administration of IFNs at the early stage prior to severe COVID-19 may elicit an autonomous antiviral state, restrict the virus infection, and prevent COVID-19 progression. Inborn genetic flaws and autoreactive antibodies that block IFN response have been significantly associated with about 14% of patients with life-threatening COVID-19 pneumonia. In most severe COVID-19 patients without genetic errors in IFN-relevant gene loci, IFN dysregulation is progressively worsened and associated with the situation of pro-inflammation and immunopathy, which is prone to autoimmunity. In addition, the high correlation of severe COVID-19 with seniority, males, and individuals with pre-existing comorbidities will be plausibly explained by the coincidence of IFN aberrance in these situations. Collectively, current studies call for a better understanding of the IFN response regarding the spatiotemporal determination and subtype-specificity against SARS-CoV-2 infections, which are warranted to devise IFN-related prophylactics and therapies. -

Interplay Between Hepatitis D Virus and the Interferon Response
viruses Review Interplay between Hepatitis D Virus and the Interferon Response Zhenfeng Zhang 1 and Stephan Urban 1,2,* 1 Department of Infectious Diseases, Molecular Virology, University Hospital Heidelberg, 69120 Heidelberg, Germany; [email protected] 2 German Centre for Infection Research (DZIF), Partner Site Heidelberg, 69120 Heidelberg, Germany * Correspondence: [email protected]; Tel.: +49-6221-564-902 Received: 27 October 2020; Accepted: 18 November 2020; Published: 20 November 2020 Abstract: Chronic hepatitis D (CHD) is the most severe form of viral hepatitis, with rapid progression of liver-related diseases and high rates of development of hepatocellular carcinoma. The causative agent, hepatitis D virus (HDV), contains a small (approximately 1.7 kb) highly self-pairing single-strand circular RNA genome that assembles with the HDV antigen to form a ribonucleoprotein (RNP) complex. HDV depends on hepatitis B virus (HBV) envelope proteins for envelopment and de novo hepatocyte entry; however, its intracellular RNA replication is autonomous. In addition, HDV can amplify HBV independently through cell division. Cellular innate immune responses, mainly interferon (IFN) response, are crucial for controlling invading viruses, while viruses counteract these responses to favor their propagation. In contrast to HBV, HDV activates profound IFN response through the melanoma differentiation antigen 5 (MDA5) pathway. This cellular response efficiently suppresses cell-division-mediated HDV spread and, to some extent, early stages of HDV de novo infection, but only marginally impairs RNA replication in resting hepatocytes. In this review, we summarize the current knowledge on HDV structure, replication, and persistence and subsequently focus on the interplay between HDV and IFN response, including IFN activation, sensing, antiviral effects, and viral countermeasures. -

Estimating the Long-Term Clinical and Economic Outcomes of Daclatasvir
VALUE IN HEALTH REGIONAL ISSUES ] ( ]]]]) ]]]– ]]] Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/vhri Estimating the Long-Term Clinical and Economic Outcomes of Daclatasvir Plus Asunaprevir in Difficult-to-Treat Japanese Patients Chronically Infected with Hepatitis C Genotype 1b Phil McEwan, PhD1,2, Thomas Ward, MSc1,*, Samantha Webster, BSc1, Yong Yuan, PhD3, Anupama Kalsekar, MS3, Kristine Broglio, MS4, Isao Kamae, PhD5, Melanie Quintana, PhD4, Scott M. Berry, PhD4, Masahiro Kobayashi, MD6, Sachie Inoue, PhD7, Ann Tang, PhD8, Hiromitsu Kumada, MD6 1Health Economics and Outcomes Research Ltd., Monmouth, UK; 2Centre for Health Economics, Swansea University, Swansea, UK; 3Global Health Economics and Outcomes Research, Princeton, NJ, USA; 4Berry Consultants, LLC, Austin, TX, USA; 5Graduate School of Public Policy, University of Tokyo, Tokyo, Japan; 6Department of Hepatology, Toranomon Hospital, Tokyo, Japan; 7CRECON Research and Consulting, Inc., Tokyo, Japan; 8Health Economics and Outcomes Research, Bristol-Myers K.K., Tokyo, Japan ABSTRACT Objectives: Japan has one of the highest endemic rates of hepatitis C treated with TVR þ pegIFN-α/RBV. Results: Initiating DCV þ ASV virus (HCV) infection. Treatments in Japan are currently limited to treatment in patients in the chronic hepatitis C disease stage resulted interferon-alfa–based regimens, which are associated with tolerability in quality-adjusted life-year gains of 0.96 and 0.77 over TVR þ pegIFN- and efficacy issues. A novel regimen combining two oral HCV α/RBV for NRs and PRs, respectively, and a gain of 2.61 in interferon- therapies, daclatasvir and asunaprevir (DCV þ ASV), has shown alfa–ineligible/intolerant patients over no treatment. -

Pegasys, INN-Peginterferon Alfa-2A
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion and scientific discussion on procedures, which have been finalised before 1 April 2005. For scientific information on procedures after this date please refer to module 8B. 1. Introduction Peginterferon alfa-2a is a polyethylene glycol (PEG)-modified form of human recombinant interferon alfa-2a intended for the treatment of adult patients with chronic hepatitis C (CHC) or chronic hepatitis B (CHB). Chronic hepatitis C is a major public health problem: hepatitis C virus (HCV) is responsible for a large proportion of chronic liver disease, accounting for 70% of cases of chronic hepatitis in industrialised countries. Globally there are an estimated 150 million chronic carriers of the virus, including 5 million in Western Europe. Without treatment approximately 30% of those infected with HCV will develop cirrhosis over a time frame of 30 years or more. For those with HCV-related cirrhosis, the prognosis is poor – a significant proportion will develop a life-threatening complication (either decompensated liver disease or an hepatocellular carcinoma) within a few years. The only therapy for those with advanced cirrhosis is liver transplantation, which carries a high mortality. In those who survive transplantation, viral recurrence in the new liver is almost inevitable and a significant proportion of infected liver grafts develop a progressive fibrosis that leads to recurrence of cirrhosis within 5 years. Interferon alfa monotherapy has been shown to be effective for the treatment of chronic hepatitis although sustained response rates occurred in approximately 15 to 30 % of patients treated for long duration (12-18 months). The current reference therapy is interferon alpha in combination with ribavirin, which resulted in an increase in biochemical and virological sustained response rates to approximately 40 % in naïve patients. -

Alfa Interferons
Clinical Pharmacy Program Guidelines for Alfa Interferons Program Prior Authorization Medication Intron A (interferon alfa-2b), Pegasys (peginterferon alfa-2a), PegIntron and Sylatron™ (peginterferon alfa-2b) Markets in Scope California,Colorado,Hawaii, Maryland, Nevada, New Jersey, New York, New York EPP, Pennsylvania- CHIP, Rhode Island, South Carolina Issue Date 9/2009 Pharmacy and 11/2020 Therapeutics Approval Date Effective Date 12/2020 1. Background: Indications Intron A (interferon alfa-2b) is indicated for the treatment of chronic hepatitis C in patients 18 years of age or older with compensated liver disease who have a history of blood or blood- product exposure and/or are HCV antibody positive. Intron A has additional FDA labeling for the treatment of chronic hepatitis C in patients 3 years of age and older with compensated liver disease previously untreated with alpha interferon therapy and in patients 18 years of age and older who have relapsed following alpha interferon therapy. Intron A is also indicated for the treatment of chronic hepatitis B in patients 1 year of age or older with compensated liver disease. Patients who have been serum HBsAg positive for at least 6 months and have evidence of HBV replication (serum HBeAg positive) with elevated serum ALT are candidates for treatment. Intron A is indicated for the treatment of patients 18 years of age or older with hairy cell leukemia. Intron A is indicated as adjuvant to surgical treatment in patients 18 years of age or older with malignant melanoma who are free of disease but a high risk for systemic recurrence, within 56 days of surgery. -

Induces Antigen Presentation in B Cells Cell-Activating Factor of The
B Cell Maturation Antigen, the Receptor for a Proliferation-Inducing Ligand and B Cell-Activating Factor of the TNF Family, Induces Antigen Presentation in B Cells This information is current as of September 27, 2021. Min Yang, Hidenori Hase, Diana Legarda-Addison, Leena Varughese, Brian Seed and Adrian T. Ting J Immunol 2005; 175:2814-2824; ; doi: 10.4049/jimmunol.175.5.2814 http://www.jimmunol.org/content/175/5/2814 Downloaded from References This article cites 54 articles, 36 of which you can access for free at: http://www.jimmunol.org/content/175/5/2814.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 27, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology B Cell Maturation Antigen, the Receptor for a Proliferation-Inducing Ligand and B Cell-Activating Factor of the TNF Family, Induces Antigen Presentation in B Cells1 Min Yang,* Hidenori Hase,* Diana Legarda-Addison,* Leena Varughese,* Brian Seed,† and Adrian T. -
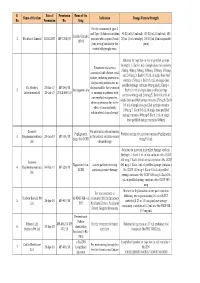
Final List of R-DNA Based Drugs Approved in the Country.Xlsx
S. Date of Permission Name of the Name of the firm Indication Dosage Form & Strength No. Permission No. Drug For the treatment of type -I and Type -II diabetes mellitus 40 IU/ml (10 ml vial), 100 IU/ml (10 ml vial), 100 Insulin Glargine 1 Wockhardt Limited 22.02.2007 MF-7206/07 patients who required basal IU/ml (3 ml cartridge), 100 IU/ml (3 ml disposable 100 IU (long acting) insulin for the pens) control of hyperglycemia. Solution for injection in vial or prefilled syringe Strength: 1. Each 1 mL of single dose vial contains Treatment of anaemia 25mcg, 40mcg, 60mcg, 100mcg, 200mcg, 300mcg associated with chronic renal and 500mcg 2. Each 0.75 mL of single dose vial failure, including patients on contains 150mcg 3. Each 0.3 mL of single dose dialysis and patients not on prefilled syringe contains 60mcg and 150mcg 4. Dr. Reddy's 23-Mar-10 MF-246/10 dialysis and for the treatment 2 Darbepoetin alfa Each 0.4 mL of single dose prefilled syringe Laboratories Ltd. 20-Jul-10 BULK-668/10 of anaemia in patients with contains 40mcg and 200mcg 5. Each 0.42 mL of non-myeloid malignancies, single dose prefilled syringe contains 25mcg 6. Each where anaemia is due to the 0.5 mL of single dose prefilled syringe contains effect of concomitantly 100mcg 7. Each 0.6 mL of single dose prefilled administered chemotherapy syringe contains 300mcg 8. Each 1 mL of single dose prefilled syringe contains 500mcg Gennova For prevention of neutropenia Pegfilgrastim Prefilled syringe for injection contains Pegfilgrastim 3 Biopharmaceuticals 29-Jan-10 MF-104/10 in the patient receiving cancer (peg-r-hu-GCSF) 6 mg/0.6 mL Ltd.