The Who's Who of Plant Viruses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Requirements for the Packaging of Geminivirus Circular Single-Stranded DNA: Effect of DNA Length and Coat Protein Sequence
viruses Article Requirements for the Packaging of Geminivirus Circular Single-Stranded DNA: Effect of DNA Length and Coat Protein Sequence Keith Saunders 1,* , Jake Richardson 2, David M. Lawson 1 and George P. Lomonossoff 1 1 Department of Biological Chemistry, John Innes Centre, Norwich Research Park, Norwich NR4 7UH, UK; [email protected] (D.M.L.); george.lomonossoff@jic.ac.uk (G.P.L.) 2 Department of Cell and Developmental Biology, John Innes Centre, Norwich Research Park, Norwich NR4 7UH, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-1603-450733 Received: 10 September 2020; Accepted: 28 October 2020; Published: 30 October 2020 Abstract: Geminivirus particles, consisting of a pair of twinned isometric structures, have one of the most distinctive capsids in the virological world. Until recently, there was little information as to how these structures are generated. To address this, we developed a system to produce capsid structures following the delivery of geminivirus coat protein and replicating circular single-stranded DNA (cssDNA) by the infiltration of gene constructs into plant leaves. The transencapsidation of cssDNA of the Begomovirus genus by coat protein of different geminivirus genera was shown to occur with full-length but not half-length molecules. Double capsid structures, distinct from geminate capsid structures, were also generated in this expression system. By increasing the length of the encapsidated cssDNA, triple geminate capsid structures, consisting of straight, bent and condensed forms were generated. The straight geminate triple structures generated were similar in morphology to those recorded for a potato-infecting virus from Peru. -

Beet Curly Top Virus Strains Associated with Sugar Beet in Idaho, Oregon, and a Western U.S
Plant Disease • 2017 • 101:1373-1382 • http://dx.doi.org/10.1094/PDIS-03-17-0381-RE Beet curly top virus Strains Associated with Sugar Beet in Idaho, Oregon, and a Western U.S. Collection Carl A. Strausbaugh and Imad A. Eujayl, United States Department of Agriculture–Agricultural Research Service (USDA-ARS) Northwest Irrigation and Soils Research Laboratory, Kimberly, ID 83341; and William M. Wintermantel, USDA-ARS, Salinas, CA 93905 Abstract Curly top of sugar beet is a serious, yield-limiting disease in semiarid pro- Logan) strains and primers that amplified a group of Worland (Wor)- duction areas caused by Beet curly top virus (BCTV) and transmitted like strains. The BCTV strain distribution averaged 2% Svr, 30% CA/ by the beet leafhopper. One of the primary means of control for BCTV Logan, and 87% Wor-like (16% had mixed infections), which differed in sugar beet is host resistance but effectiveness of resistance can vary from the previously published 2006-to-2007 collection (87% Svr, 7% among BCTV strains. Strain prevalence among BCTV populations CA/Logan, and 60% Wor-like; 59% mixed infections) based on a contin- was last investigated in Idaho and Oregon during a 2006-to-2007 collec- gency test (P < 0.0001). Whole-genome sequencing (GenBank acces- tion but changes in disease severity suggested a need for reevaluation. sions KT276895 to KT276920 and KX867015 to KX867057) with Therefore, 406 leaf samples symptomatic for curly top were collected overlapping primers found that the Wor-like strains included Wor, Colo- from sugar beet plants in commercial sugar beet fields in Idaho and rado and a previously undescribed strain designated Kimberly1. -

SUGARBEET S P R I N G I S S UE
THE SUGARBEET S P R I N G ISS UE SPRING 2011 SUGARBEET N EWS LETTER he Sugarbeet is published by The Amalgamated Sugar Company. The magazine is prepared by the Agriculture TDepartment to provide growers with up-to-date information on growing and harvesting sugarbeets. The magazine is also published to help upgrade the standards of the U.S. beet industry by providing a reliable source of information for agronomists, scientists, sugar company personnel, students, and others interested in this vital food crop. Articles appearing in The Sugarbeet, with the exception of those items credited to other sources, may be quoted or reprinted without permission; however, mention of this publication is requested when material herein is re- printed. Although every effort is made to ensure that the material is accurate, no responsibility can be assumed for er- rors over which the editor has no control. Mention or illustration of methods, devices, equipment, or commercial products does not constitute an endorsement by the company. Address all communication to the Editor, The Sugarbeet, P.O. Box 8787, Nampa, ID 83653-8787. Agriculture Offices Nyssa District Nyssa, Oregon Mini-Cassia District Paul, Idaho Agriculture Research Offices Twin Falls, Idaho Twin Falls District Twin Falls, Idaho Technical Advisor John Schorr Elwyhee District Corporate Director of Agriculture Mountain Home, Idaho Editor Nampa District Dennis Searle Nampa, Idaho Ag Services Manager SPRING ISSUE 2011 CONTENTS Mini-Cassia District - 2010 ........................................... 2 Nampa District - 2010 .............................................. 4 Twin Falls District - 2010 ............................................ 5 Elwyhee District - 2010 ............................................. 6 Washington District - 2010 .......................................... 6 Nyssa District - 2010 ............................................... 7 Controlling Severe Curly Top In Sugarbeet .............................. -

Hemp Pests Presentation Lukas
Hemp Production Considerations -Insects and Diseases- Scott Lukas Assistant Professor Hermiston Agricultural Research & Extension Center What is hemp vs. marijuana? Cannabis sativa Hemp Marijuana ≤ 0.3% Total THC* > 0.3% Total THC* * The first American flag made by Betsy Ross was made from industrial hemp 1777 Where are we with hemp 2019 Oregon production • 63,000 registered acres in 2019, nearly six times more than in 2018 • 1,940 registered growers in the state • Most all of the crop is being grown for hemp essential oils with dependence on feminized seeds for production Expansive production and limited research, we are all learning at the same time. 1. Overview of insect pests that prey on or potentially may affect hemp 2. Diseases observed in 2019 hemp crops I will provide some management options but cannot list products or specific control options Products are under development and approval Research to support insect and disease control is underway Insects associated with hemp Group I: Below soil Group II: Leaf Group III: Stem/stalk Group IV: Flowers and seeds Wireworm Pacific coast wireworm Limonius canus Click beetle larvae Determine levels Bait stations Soil collection – sieve Soil inspection during tillage Will weaken or kill plants from damage or secondary infection Wireworm Pacific coast wireworm Life Cycle Move upward in soil in spring - Overwinter at 12”-24” depth Wireworm Group II – Leaf feeders Sucking and piercing Chewing (Leaf defoliators) Sucking & Piercing Leafhoppers Spider Mites Aphids Thrips Russet Mites CSU-W Cranshaw -

STUDIES on PROPERTIES OP the CURLY TOP VIRUS ' by C
STUDIES ON PROPERTIES OP THE CURLY TOP VIRUS ' By C. W. BENNETT 2 Pathologist, Division of Sugar Plant Investigations, Bureau of Plant Industry¡ United States Department of Agriculture INTRODUCTION The determination of properties of the curly top virus is somewhat more difficult than are similar determinations with many other vi- ruses, as the percentage infection from artificial inoculation is too low to afford a critical test for the presence or absence of virus. The unsatisfactory results from artificial inoculations have forced investigators to rely on the natural insect vector, Eutettix tenellus (Baker), for the production of any considerable amount of infection. Experimental work on the properties of the curly top virus was facilitated by the development of a method by Carter (5) ^ for arti- ficially feeding the beet leaf hopper. This method consists in plac- ing a liquid containing the virus in a bag made of animal membrane and allowing nonviruliferous leaf hoppers access to the outside of the bag. The leaf hoppers puncture this membrane and feed sufficiently on the liquid to acquire the virus. In this way the beet leaf hopper may be utilized to transfer virus from the liquid medium to suscep- tible tissue of beet plants. Modifications of Carteras original method of feeding the beet leaf hopper have been devised and used with considerable success in studies on the properties of the virus by those interested in this field of research. Severin and Swezy (14) determined that the virus is filterable, and recently Severin and Freitag (13) published results of further investigations on properties of the virus. -

Controlling Curly Top Virus of Tomato
September 2014 Horticulture/PlantProblems/2014-01pr Controlling Curly Top Virus of Tomato Rick Heflebower, Horticulture Agent, Utah State University Extension, and Jeff Schalau, Agriculture Agent, University of Arizona Extension Introduction before it can be transmitted. Once incubated, the BLH transmits the virus to other plants during Beet Curly Top Virus (BCTV) is responsible for the feeding. The BLHs have a piercing-sucking feeding disease known as “Curly Top of Tomato.” This habit, and they inject and leave behind virus virus can infect a wide range of host plants and particles inside the plant. BLHs carrying the virus usually occurs in semiarid areas in western North need only to feed for 1 minute on an uninfected America, including New Mexico, Utah, California, plant to transmit the virus. The disease is Washington, and Oregon (Damicone & Grantham, transported within the plant through the phloem 2003). Beet Leaf Hopper (BLH) transmits the tissue. Symptoms usually begin to appear after 24 disease to a wide variety of plants, including more hours in hot temperatures and progress more slowly than 300 species of dicotyledons (broad-leaf plants). in cooler temperatures (Schalau, 2012). BLHs that have acquired BCTV can transmit the virus for the Monocotyledonous hosts (typically grasses) have remainder of their life; however, the number of not been reported nor have there been reports of the plants infected decreases when the insects are not disease being transmitted in seed. Crop plants continually or frequently feeding on infected plants. affected by this virus include beets, tomatoes, Swiss chard, spinach, beans and cucurbits such as watermelon, cucumbers and squash. -

HTS-Based Diagnostics of Sugarcane Viruses: Seasonal Variation and Its Implications for Accurate Detection
viruses Article HTS-Based Diagnostics of Sugarcane Viruses: Seasonal Variation and Its Implications for Accurate Detection Martha Malapi-Wight 1,*,† , Bishwo Adhikari 1 , Jing Zhou 2, Leticia Hendrickson 1, Clarissa J. Maroon-Lango 3, Clint McFarland 4, Joseph A. Foster 1 and Oscar P. Hurtado-Gonzales 1,* 1 USDA-APHIS Plant Germplasm Quarantine Program, Beltsville, MD 20705, USA; [email protected] (B.A.); [email protected] (L.H.); [email protected] (J.A.F.) 2 Department of Agriculture, Agribusiness, Environmental Sciences, Texas A&M University-Kingsville, Kingsville, TX 78363, USA; [email protected] 3 USDA-APHIS-PPQ-Emergency and Domestic Program, Riverdale, MD 20737, USA; [email protected] 4 USDA-APHIS-PPQ-Field Operations, Raleigh, NC 27606, USA; [email protected] * Correspondence: [email protected] (M.M.-W.); [email protected] (O.P.H.-G.) † Current affiliation: USDA-APHIS BRS Biotechnology Risk Analysis Program, Riverdale, MD 20737, USA. Abstract: Rapid global germplasm trade has increased concern about the spread of plant pathogens and pests across borders that could become established, affecting agriculture and environment systems. Viral pathogens are of particular concern due to their difficulty to control once established. A comprehensive diagnostic platform that accurately detects both known and unknown virus species, as well as unreported variants, is playing a pivotal role across plant germplasm quarantine programs. Here we propose the addition of high-throughput sequencing (HTS) from total RNA to the routine Citation: Malapi-Wight, M.; quarantine diagnostic workflow of sugarcane viruses. We evaluated the impact of sequencing depth Adhikari, B.; Zhou, J.; Hendrickson, needed for the HTS-based identification of seven regulated sugarcane RNA/DNA viruses across L.; Maroon-Lango, C.J.; McFarland, two different growing seasons (spring and fall). -

Seed Transmission of Beet Curly Top Virus and Beet Curly Top Iran Virus in a Local Cultivar of Petunia in Iran
viruses Article Seed Transmission of Beet Curly Top Virus and Beet Curly Top Iran Virus in a Local Cultivar of Petunia in Iran Ameneh Anabestani 1, Seyed Ali Akbar Behjatnia 1,*, Keramat Izadpanah 1, Saeid Tabein 1 and Gian Paolo Accotto 2 ID 1 Plant Virology Research Center, College of Agriculture, Shiraz University, Shiraz 71441-65186, Iran; [email protected] (A.A.); [email protected] (K.I.); [email protected] (S.T.) 2 Institute for Sustainable Plant Protection, National Research Council of Italy (IPSP-CNR), 10135 Torino, Italy; [email protected] * Correspondence: [email protected]; Tel.: +98-917-306-7743 Received: 12 September 2017; Accepted: 13 October 2017; Published: 16 October 2017 Abstract: Beet curly top virus (BCTV) and beet curly top Iran virus (BCTIV) are known as the causal agents of curly top disease in beet and several other dicotyledonous plants in Iran. These viruses are transmitted by Circulifer species, and until now, there has been no confirmed report of their seed transmission. A percentage (38.2–78.0%) of the seedlings developed from the seeds of a petunia local cultivar under insect-free conditions showed stunting, interveinal chlorosis, leaf curling, and vein swelling symptoms, and were infected by BCTV when tested by PCR. Presence of BCTV in seed extracts of petunia local cultivar was confirmed by PCR and IC-PCR, followed by sequencing. Agroinoculation of curly top free petunia plants with a BCTV infectious clone resulted in BCTV infection of plants and their developed seeds. These results show the seed infection and transmission of BCTV in a local cultivar of petunia. -

Interaction Between the Chromatin of Beet Curly Top Virus and TFL2 Protein
Interaction between the chromatin of Beet curly top virus and TFL2 protein Sara Hosseini Independent project in biology-Master’s thesis, 30 hp, EX0565 Swedish University of Agricultural Sciences Department of Plant Biology and Forest Genetics Uppsala 2010 Nr: 107 ISSN: 1651-5196 Plant Biology-Master’s programme Interaction between the chromatin of Beet curly top virus and TFL2 protein Interaktion mellan Beet curly top virus’ kromatin och TFL2-protein Sara Hosseini Supervisors: Anders Kvarnheden Department of Plant Biology and Forest Genetics Swedish University of Agricultural Sciences (SLU) PO Box 7080, 750 07 Uppsala Annika Sundås-Larsson Department of Evolution, Genomics and Systematics Physiological Botany Uppsala University PO Box Norbyvägen 18D, 752 36 Uppsala Examiner: Sarosh Bejai Department of Plant Biology and Forest Genetics, Swedish University of Agricultural Sciences (SLU) PO Box 7080, 750 07, Uppsala Key words: Euchromatin: The lightly packed form of chromatin that is often under active transcription. Geminiviruses: A devastating group of plant viruses which have single-stranded DNA genomes. Histone: The proteins found in eukaryotic cell nuclei which package the DNA into nucleosomes. Swedish University of Agricultural Sciences Faculty of Natural Resources and Agricultural Sciences Department of Plant Biology and Forest Genetics Master’s thesis in Plant Biology, EX0565, 30 hp, Advanced E Uppsala 2010 Nr: 107 ISSN: 1651-5196 http://stud.epsilon.slu.se Plant Biology-Master’ s Programme ABSTRACT: Beet curly top virus (BCTV) is one of the most devastating DNA viruses, causing curly top diseases in a wide range of plants. This virus belongs to the family Geminiviridae, which replicate their circular single-stranded DNA genomes in the plant cell’s nucleus through employing host replication factors. -

CHARACTERIZATION of TWO BEGOMOVIRUSES ISOLATED from Sida Santaremensis Monteiro and Sida Acuta Burm. F by HAMED ADNAN AL-AQEEL A
CHARACTERIZATION OF TWO BEGOMOVIRUSES ISOLATED FROM Sida santaremensis Monteiro AND Sida acuta Burm. f By HAMED ADNAN AL-AQEEL A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 Copyright 2003 by Hamed Adnan Al-Aqeel This dedicated to my family my father Dr. Adnan, my mother Fareda and my wife Hanin. TABLE OF CONTENTS page LIST OF TABLES............................................................................................................. vi LIST OF FIGURES .......................................................................................................... vii ABSTRACT....................................................................................................................... ix CHAPTER 1 HISTORY AND LITERATURE REVIEW .................................................................1 Geminivirus History .....................................................................................................1 Taxonomy and Nucleotide Functions...........................................................................3 Begomoviruses .............................................................................................................5 The Genus Sida.............................................................................................................6 Viruses Infecting Sida spp............................................................................................7 Begomoviruses -

Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: a Review
plants Review Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review Richard Hanˇcinský 1, Daniel Mihálik 1,2,3, Michaela Mrkvová 1, Thierry Candresse 4 and Miroslav Glasa 1,5,* 1 Faculty of Natural Sciences, University of Ss. Cyril and Methodius, Nám. J. Herdu 2, 91701 Trnava, Slovakia; [email protected] (R.H.); [email protected] (D.M.); [email protected] (M.M.) 2 Institute of High Mountain Biology, University of Žilina, Univerzitná 8215/1, 01026 Žilina, Slovakia 3 National Agricultural and Food Centre, Research Institute of Plant Production, Bratislavská cesta 122, 92168 Piešt’any, Slovakia 4 INRAE, University Bordeaux, UMR BFP, 33140 Villenave d’Ornon, France; [email protected] 5 Biomedical Research Center of the Slovak Academy of Sciences, Institute of Virology, Dúbravská cesta 9, 84505 Bratislava, Slovakia * Correspondence: [email protected]; Tel.: +421-2-5930-2447 Received: 16 April 2020; Accepted: 22 May 2020; Published: 25 May 2020 Abstract: Plant viruses infecting crop species are causing long-lasting economic losses and are endangering food security worldwide. Ongoing events, such as climate change, changes in agricultural practices, globalization of markets or changes in plant virus vector populations, are affecting plant virus life cycles. Because farmer’s fields are part of the larger environment, the role of wild plant species in plant virus life cycles can provide information about underlying processes during virus transmission and spread. This review focuses on the Solanaceae family, which contains thousands of species growing all around the world, including crop species, wild flora and model plants for genetic research. -
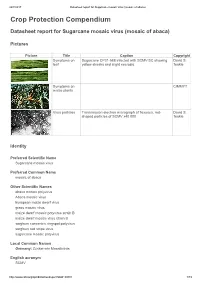
Datasheet Report for Sugarcane Mosaic Virus (Mosaic of Abaca)
24/7/2017 Datasheet report for Sugarcane mosaic virus (mosaic of abaca) Crop Protection Compendium Datasheet report for Sugarcane mosaic virus (mosaic of abaca) Pictures Picture Title Caption Copyright Symptoms on Sugarcane CP31-588 infected with SCMV-SC showing David S. leaf yellow streaks and slight necrosis. Teakle Symptoms on CIMMYT maize plants Virus particles Transmission electron micrograph of flexuous, rod- David S. shaped particles of SCMV x40 000. Teakle Identity Preferred Scientific Name Sugarcane mosaic virus Preferred Common Name mosaic of abaca Other Scientific Names abaca mosaic potyvirus Abaca mosaic virus European maize dwarf virus grass mosaic virus maize dwarf mosaic potyvirus strain B maize dwarf mosaic virus strain B sorghum concentric ringspot potyvirus sorghum red stripe virus sugarcane mosaic potyvirus Local Common Names Germany: Zuckerrohr Mosaikvirus English acronym SCMV http://www.cabi.org/cpc/datasheetreport?dsid=49801 1/19 24/7/2017 Datasheet report for Sugarcane mosaic virus (mosaic of abaca) EPPO code SCMV00 (Sugarcane mosaic potyvirus) Taxonomic Tree Domain: Virus Group: "Positive sense ssRNA viruses" Group: "RNA viruses" Family: Potyviridae Genus: Potyvirus Species: Sugarcane mosaic virus Notes on Taxonomy and Nomenclature This virus was described from sugarcane by Brandes (1919). Strains naturally infecting certain other hosts are also known as abaca mosaic potyvirus (Eloja and Tinsley, 1963) and as maize dwarf mosaic potyvirus strain B (Mackenzie et al., 1966; Louie and Knoke, 1975). Until recently, most aphid-borne potyviruses infecting sugarcane and other members of the Poaceae were assumed to be either sugarcane mosaic virus or maize dwarf mosaic virus. However, new criteria for speciation of these members of the Potyviridae have been proposed (Shukla et al., 1989; Shukla et al., 1992; Shukla and Ward, 1994): the amino-acid sequence homology of their coat proteins, and serological relationships with antisera derived from epitopes in the amino terminus rather than the core region of the coat protein.