Biotechnological Applications to Sugarcane Pathogens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HTS-Based Diagnostics of Sugarcane Viruses: Seasonal Variation and Its Implications for Accurate Detection
viruses Article HTS-Based Diagnostics of Sugarcane Viruses: Seasonal Variation and Its Implications for Accurate Detection Martha Malapi-Wight 1,*,† , Bishwo Adhikari 1 , Jing Zhou 2, Leticia Hendrickson 1, Clarissa J. Maroon-Lango 3, Clint McFarland 4, Joseph A. Foster 1 and Oscar P. Hurtado-Gonzales 1,* 1 USDA-APHIS Plant Germplasm Quarantine Program, Beltsville, MD 20705, USA; [email protected] (B.A.); [email protected] (L.H.); [email protected] (J.A.F.) 2 Department of Agriculture, Agribusiness, Environmental Sciences, Texas A&M University-Kingsville, Kingsville, TX 78363, USA; [email protected] 3 USDA-APHIS-PPQ-Emergency and Domestic Program, Riverdale, MD 20737, USA; [email protected] 4 USDA-APHIS-PPQ-Field Operations, Raleigh, NC 27606, USA; [email protected] * Correspondence: [email protected] (M.M.-W.); [email protected] (O.P.H.-G.) † Current affiliation: USDA-APHIS BRS Biotechnology Risk Analysis Program, Riverdale, MD 20737, USA. Abstract: Rapid global germplasm trade has increased concern about the spread of plant pathogens and pests across borders that could become established, affecting agriculture and environment systems. Viral pathogens are of particular concern due to their difficulty to control once established. A comprehensive diagnostic platform that accurately detects both known and unknown virus species, as well as unreported variants, is playing a pivotal role across plant germplasm quarantine programs. Here we propose the addition of high-throughput sequencing (HTS) from total RNA to the routine Citation: Malapi-Wight, M.; quarantine diagnostic workflow of sugarcane viruses. We evaluated the impact of sequencing depth Adhikari, B.; Zhou, J.; Hendrickson, needed for the HTS-based identification of seven regulated sugarcane RNA/DNA viruses across L.; Maroon-Lango, C.J.; McFarland, two different growing seasons (spring and fall). -
Datasheet Report for Sugarcane Mosaic Virus (Mosaic of Abaca)
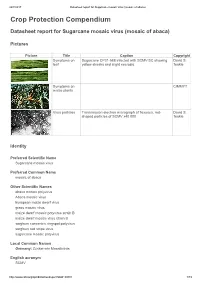
24/7/2017 Datasheet report for Sugarcane mosaic virus (mosaic of abaca) Crop Protection Compendium Datasheet report for Sugarcane mosaic virus (mosaic of abaca) Pictures Picture Title Caption Copyright Symptoms on Sugarcane CP31-588 infected with SCMV-SC showing David S. leaf yellow streaks and slight necrosis. Teakle Symptoms on CIMMYT maize plants Virus particles Transmission electron micrograph of flexuous, rod- David S. shaped particles of SCMV x40 000. Teakle Identity Preferred Scientific Name Sugarcane mosaic virus Preferred Common Name mosaic of abaca Other Scientific Names abaca mosaic potyvirus Abaca mosaic virus European maize dwarf virus grass mosaic virus maize dwarf mosaic potyvirus strain B maize dwarf mosaic virus strain B sorghum concentric ringspot potyvirus sorghum red stripe virus sugarcane mosaic potyvirus Local Common Names Germany: Zuckerrohr Mosaikvirus English acronym SCMV http://www.cabi.org/cpc/datasheetreport?dsid=49801 1/19 24/7/2017 Datasheet report for Sugarcane mosaic virus (mosaic of abaca) EPPO code SCMV00 (Sugarcane mosaic potyvirus) Taxonomic Tree Domain: Virus Group: "Positive sense ssRNA viruses" Group: "RNA viruses" Family: Potyviridae Genus: Potyvirus Species: Sugarcane mosaic virus Notes on Taxonomy and Nomenclature This virus was described from sugarcane by Brandes (1919). Strains naturally infecting certain other hosts are also known as abaca mosaic potyvirus (Eloja and Tinsley, 1963) and as maize dwarf mosaic potyvirus strain B (Mackenzie et al., 1966; Louie and Knoke, 1975). Until recently, most aphid-borne potyviruses infecting sugarcane and other members of the Poaceae were assumed to be either sugarcane mosaic virus or maize dwarf mosaic virus. However, new criteria for speciation of these members of the Potyviridae have been proposed (Shukla et al., 1989; Shukla et al., 1992; Shukla and Ward, 1994): the amino-acid sequence homology of their coat proteins, and serological relationships with antisera derived from epitopes in the amino terminus rather than the core region of the coat protein. -

Virus Diseases and Crop Biosecurity
Virus Diseases and Crop Biosecurity NATO Security through Science Series This Series presents the results of scientific meetings supported under the NATO Programme for Security through Science (STS) Meetings supported by the NATO STS Programme are in security-related priority areas of Defence Against Terrorism or Countering Other Threats to Security. The types of meeting supported are generally “Advanced Study Institute” and “Advanced Research Workshops”. The NATO STS Series collects together the results of these meetings. The meetings are co-organized by scientist from NATO countries and scientists from NATO’s “Partner” or “Mediterranean Dialogue” countries. The observations and recommendations made at the meetings, as well as the contents of the volumes in the Series, reflect those of participants and contributors only; they should not necessarily be regarded as reflecting NATO views or policy. Advanced Study Institutes (ASI) are high-level tutorial courses to convey the latest developments in a subject to an advanced-level audience Advanced Research Workshops (ARW) are expert meetings where an intense but informal exchange of views at the frontiers of a subject aims at identifying directions for future actions Following a transformation of the programme in 2004 the Series has been re-named and re-organised. Recent volumes on topics not related to security, which result from meetings supported under the programme earlier, may be found in the NATO Science Series. The Series is published by IOS Press, Amsterdam, and Springer, Dordrecht, in conjunction with the NATO Public Diplomacy Division. Sub-Series A. Chemistry and Biology Springer B. Physics and Biophysics Springer C. Environmental Security Springer D. -

Molecular Characterization of a Novel Segmented Dsrna Mycovirus and Its Association with Hypovirulence of Fusarium Graminearum
Molecular characterization of a novel segmented dsRNA mycovirus and its association with hypovirulence of Fusarium graminearum. Dissertation A thesis submitted to the Fachbereich Biologie, Universität Hamburg for the degree of doctor rerum naturalium By Darissa Omar Bethlehem, Palestine Hamburg, 2011 17 December 2010 Mr. Omar Darissa Bramfelder Chaussee 9 22177 Hamburg, Germany RE: Review of thesis entitled “Molecular characterization of a novel segmented dsRNA mycovirus and its association with hypovirulence of Fusarium graminearum” by Mr. Omar Darissa I confirm that the thesis of Mr. Omar Darissa was reviewed by me, a native English speaker, for English language accuracy. The thesis is well written, and therefore I would recommend that the dissertation be accepted in its current form. During my 42 years as a professor, I have read many theses and Mr. Darissa’s thesis meets the standards for the University of Wisconsin-Madison. Phone numbers: University of Wisconsin: 608-262-1410 Home: 608-845-7717 ([email protected] or [email protected]) To my parents Mousa and Meriam Darissa To my wife Laila Darissa To my children Mousa, Mahmoud, and Mamoun Contents Contents Contents..................................................................................................................... i List of Figures ......................................................................................................... v List of Tables ........................................................................................................ -

Influence of Maize Mosaic Virus and Host Plant Genotypes on the Bio
INFLUENCE OF MAIZE MOSAIC VIRUS AND HOST PLANT GENOTYPES ON THE BIO- ECOLOGY OF PEREGRINUS MAIDIS A THESIS SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI‘I AT MĀNOA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN ENTOMOLOGY MARCH 2012 By Clesson H. V. Higashi Thesis Committee: Alberto Bressan, Chairperson Mark G. Wright James Brewbaker TABLE OF CONTENTS ACKNOWLEDGMENTS ...............................................................................................................3 LIST OF TABLE .............................................................................................................................4 LIST OF FIGURES .........................................................................................................................5 CHAPTER 1:…. ..............................................................................................................................7 Abstract.....................................................................................................................................7 Introduction ..............................................................................................................................7 Vector biology and ecology .....................................................................................7 Maize mosaic virus ..................................................................................................8 Virus-vector interactions ........................................................................................10 -

Biosecurity Plan for the Sugarcane Industry
Biosecurity Plan for the Sugarcane Industry A shared responsibility between government and industry Version 3.0 May 2016 PLANT HEALTH AUSTRALIA | Biosecurity Plan for the Sugarcane Industry 2016 Location: Level 1 1 Phipps Close DEAKIN ACT 2600 Phone: +61 2 6215 7700 Fax: +61 2 6260 4321 E-mail: [email protected] Visit our web site: www.planthealthaustralia.com.au An electronic copy of this plan is available through the email address listed above. © Plant Health Australia Limited 2016 Copyright in this publication is owned by Plant Health Australia Limited, except when content has been provided by other contributors, in which case copyright may be owned by another person. With the exception of any material protected by a trade mark, this publication is licensed under a Creative Commons Attribution-No Derivs 3.0 Australia licence. Any use of this publication, other than as authorised under this licence or copyright law, is prohibited. http://creativecommons.org/licenses/by-nd/3.0/ - This details the relevant licence conditions, including the full legal code. This licence allows for redistribution, commercial and non-commercial, as long as it is passed along unchanged and in whole, with credit to Plant Health Australia (as below). In referencing this document, the preferred citation is: Plant Health Australia Ltd (2016) Biosecurity Plan for the Sugarcane Industry (Version 3.0 – May 2016). Plant Health Australia, Canberra, ACT. Disclaimer: The material contained in this publication is produced for general information only. It is not intended as professional advice on any particular matter. No person should act or fail to act on the basis of any material contained in this publication without first obtaining specific and independent professional advice. -
Sugarcane Germplasm Conservation and Exchange
Sugarcane Germplasm Conservation and Exchange Report of an international workshop held in Brisbane, Queensland, Australia 28-30 June 1995 Editors B.J. Croft, C.M. Piggin, E.S. Wallis and D.M. Hogarth The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. [ts mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country resean;hers in field, where Australia has special research competence. Where trade names are used thi, does not constitute endorsement of nor discrimination against any product by the Centre. This series of publications includes the full proceedings of research workshops or symposia or supported by ACIAR. Numbers in this ,,,ries are distributed internationally to individuals and scientific institutions. © Australian Centre for International Agricultural Research GPO Box 1571. Canberra, ACT 260 J Australia Croft, B.1., Piggin, CM., Wallis E.S. and Hogarth, D.M. 1996. Sugarcane germplasm conservation and exchange. ACIAR Proceedings No. 67, 134p. ISBN 1 86320 177 7 Editorial management: P.W. Lynch Typesetting and page layout: Judy Fenelon, Bawley Point. New Soulh Wales Printed by: Paragon Printers. Fyshwick, ACT Foreword ANALYSIS by the Technical Advisory Committee of the Consultative Group of Internation al Agricultural Research Centres (estimated that in 1987-88 sugar was the fourteenth most important crop in developing countries, with a gross value of production of over US$7.3 billion. In Australia it is the third most important crop, with a value in 1994-95 of around A$1.7 billion. -
Resistance to Virus Vectors in Plants the Resistvir Consortium September 2006 - O O O - Anonymous (1971)
Resistance to virus vectors in plants The ResistVir consortium September 2006 - o O o - Anonymous (1971). 11th Report of the Research Department, July 1970-June 1971. 11th Report of the Research Department, July 1970-June 1971, 90. Anonymous (1971). 1971 Research progress. Bulletin, Washington Agricultural Experiment Station, 58pp. Anonymous (1972). Annual report of the Federal Biological Institute for Agriculture and Forestry in Berlin and Braunschweig 1972. Jahresbericht 1972, 116. Anonymous (1973). Wheat. Canada, Department of Agriculture: Research branch report 1972ICrop plants, 369pp. Anonymous (1974). 20th Annual report for the year 1973. 20th Annual report for the year 1973, 93. Anonymous (1976). 22nd annual report for the year 1975. 22nd annual report for the year 1975, 111. Anonymous (1976). 76th Annual Report to the Minister for Primary Industries for the year ended June 30, 1976. 76th Annual Report to the Minister for Primary Industries for the year ended June 30, 1976, 69. Anonymous (1976). Diseases of cereals in the Mediterranean region. Poljoprivredna Znanstvena Smotra, 131-244. Anonymous (1977). 77th Annual report. 77th Annual report, 64. Anonymous (1978). 78th Annual report to the Minister for Primary Industries for the year ended June 30, 1978. Report, Bureau of Sugar Experiment Stations, Queensland, 67. Anonymous (1978). Citrus tristeza. Noticias Agricolas 8, 69-71. Anonymous (1978). Report of the planning conference on developments in the control of potato viruses, held at CIP - Lima, Peru, November 14-18, 1977. Report of the planning conference on developments in the control of potato viruses, held at CIP - Lima, Peru, November 14-18, 1977, iv. Anonymous (1980). Diseases and pests of herbage. -

The Who's Who of Plant Viruses
Vol 8, Issue 1, 2015 ISSN - 0974-2441 Review Article THE WHO’S WHO OF PLANT VIRUSES: A COGNITIVE APPROACH ANKITA LAL*, MANU PANT, ANJU RANI Department of Biotechnology, Graphic Era University, Dehradun, Uttarakhand, India. Email: [email protected] Received: 30 October 2014, Revised and Accepted: 22 November 2014 ABSTRACT Viral diseases in plants pose a serious threat to the plant production. Plant viruses are among the major factors that affect productivity and cause vast economic losses. Across the globe plant viruses persist to be a major threat to vegetation of fruits, vegetables, ornamental crops, etc. and their prevention and control are the major areas of concern. Over the past century, efforts have been put to understand the underlying mechanism of defense against plant viruses and reasons for this intercellular invasion. Resistance to plant viruses can be offered by several means such as conventional breeding methods, chemotherapy, thermotherapy and plant biotechnological interventions (plant tissue culture technology and production of transgenic plants). The present work is an attempt to understand the biology and mechanism of plant viral infections, the dangers posed by them and all possible alternatives for prevention. Keywords: Classification, Morphology, Transmission, Virus-free plant production, Genetic engineering. INTRODUCTION deficiency or pest attack resulting in a delay in the actual detection of infection. With the advent of recent biotechnological interventions Plant viruses are obligate intracellular parasites lacking molecular there has been a dramatic improvement in viral detection in plants. machinery making them unable to replicate without a host. Virus However, the problem of virus-eradication remains uncured to a large particles are immobile outside the infected host; relying on other extent. -

Job's Tears (Coix Lacryma-Jobi) As Host of Fiji Disease Virus and Perkinsiella
Job’s tears (Coix lacryma-jobi) as host of Fiji disease virus and Perkinsiella vitiensis N. S. Prasad*, B. Croft, S. Johnson and S. Work *Sugar Research Institute of Fiji, Drasa, Lautoka, Republic of Fiji Islands Email [email protected] ABSTRACT Fiji leaf gall disease is a viral disease caused by, Fiji disease virus (FDV). The virus is transmitted by an insect vector known as a planthopper (in Fiji Perkinsiella vitiensis Kirk.). The hosts of FDV in Fiji are sugarcane (Saccharum interspecific hybrid) and duruka (Saccharum edule). Duruka is a native plant food for Fijians. Fiji leaf gall is currently managed in commercial sugarcane fields by roguing and resistant varieties. Sugarcane varieties are screened for resistance using an insectary-based resistance screening method. Job’s tears (Coix lacryma-jobi L.), also known as wild maize, has been reported as a potential host of FDV and its planthopper vector. Job’s tears has been observed to develop galls similar to the characteristic symptom of Fiji leaf gall in the field. In this study, we tested Job’s tears as a host of both FDV and P. vitiensis. The results have shown that Job’s tears did not develop symptoms of Fiji leaf gall whereas sugarcane varieties including the variety, Mana, developed typical gall symptoms. Job’s tears is also not a host of Perkinsiella vitiensis. The planthoppers did not lay eggs on Job’s tears and no nymphs were observed. INTRODUCTION Fiji leaf gall (FLG) is widespread in Fiji in the commercial sugarcane crops (Saccharum interspecific hybrid), chewing canes in native gardens (Saccharum officinarum L.) and duruka (Saccharum edule Hassk.). -

Sugarcane Fiji Disease (077)
Pacific Pests, Pathogens and Weeds - Online edition Sugarcane Fiji disease (077) Summary Widespread distribution. Asia, Africa, Oceania. On sugarcane, lowland pitpit, and other Saccharum species. Occasionally, an important virus disease. Galls occur on the leaves; leaves are stiff, stunted, and flat-topped (as if chewed). Spread is by planthoppers, and in planting setts. Cultural control: use varieties bred for resistance (Fiji and Australia); do not take planting material from affected plants, even if some stalks in a stool look healthy; carefully remove diseased plants as soon as disease seen without spreading insects; collect and burn trash, after harvest. Chemical control: none recommended. Common Name Fiji disease of sugarcane; also known as Fiji leaf gall. Photo 1. Galls on the midrib or larger veins of the leaf are the first sign of infection from Scientific Name Sugarcane Fiji disease virus. Sugarcane Fiji disease fijivirus; the abbreviation is FDV. The particles are 70 um in diameter, and belong to the family Reoviridae. Photo 4. The plant on the right has a Photo 2. Stunted plant infected by Sugarcane witches' broom appearance from infection Fiji disease virus. by Sugarcane Fiji disease virus. Photo 3. Leaflets infected by Sugarcane Fiji Photo 5. Adult and nymphs of Perkinsiella disease virus are short, stiff and green and have sp., the planthopper that spreads a 'bitten-off' appearance, as if chewed by Sugarcane Fiji disease virus. cattle. AUTHO RS Helen Tsatsia & Grahame Jackson Information from SRA (2015) Information sheet ISI5001. Sugarcane Research Australia. (https://sugarresearch.com.au/w p-content/uploads/2017/03/Fiji-leaf-gall-IS13003-2.pdf); and Fiji leaf gall (2018) SRA Sugarcane Research Australia (https://sugarresearch.com.au/sugar_files/2017/03/Fiji-Gall-Leaf-Info-Sheet_2018-F.pdf); and from CABI (2012) Fiji disease virus (fiji disease of sugar cane). -

PLANT DISEASES Caused by Viruses & Virus-Like Pathogens in SAMOA & VANUATU By
SPC Techncal Paper No. 225 Surveys for PLANT DISEASES caused by Vruses & Vrus-lke pathogens n SAMOA & VANUATU By R.I. DavisA, P. MatalaveaB, M. SethC, T. MaugaB , P. JonesD A Land Resources Division, SPC – Secretariat of the Pacific Community, Private Mail Bag, Suva, Fiji Islands B Nu’u Crop Development Centre Ministry of Agriculture & Fisheries, Apia, Samoa C Vanuatu Department of Livestock and Quarantine, Private Mail Bag 95, Port Vila, Vanuatu D Plant–Pathogen Interactions Division, Rothamsted Research, Harpenden, Hertfordshire, AL5 2JQ, UK Published with financial assistance from European Union SPC Land Resources Dvson Suva, Fj May 2006 © Copyright Secretariat of the Pacific Community 2006 All rights for commercial / for profit reproduction or translation, in any form, reserved. SPC authorizes the partial reproduction or translation of this material for scientific, educational or research purposes, provided that SPC and the source document are properly acknowledged. Permission to reproduce the document and/or translate in whole, in any form, whether for commercial / for profit or non-profit purposes, must be requested in writing. Original SPC artwork may not be altered or separately published without permission. Orgnal text: Englsh Secretariat of the Pacific Community Cataloguing-in-publication data Surveys for plant dseases caused by vruses and vrus-lke pathogens n Samoa and Vanuatu / R.I. Davis, P. Matalavea, M. Seth, T. Mauga, P. Jones (Techncal Paper; 225) 1. Virus diseases of plants - Samoa - Surveys 2. Virus diseases of plants – Vanuatu – Surveys I. Title . Secretariat of the Pacific Community. III. Series 632.3 AACR2 ISSN 0081-2862 ISBN 982-00-152-8 Production: LRD Information, Communication & Extension (ICE) - Theme Group Printed by: Quality Print Limited Suva CONTENTS Abstract ...................................................................................