052898 Eosinophilia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eosinophilic Cellulitis (Wells Syndrome): a Case Report
International Journal of Research in Dermatology Baabdullah AM et al. Int J Res Dermatol. 2021 May;7(3):450-453 http://www.ijord.com DOI: https://dx.doi.org/10.18203/issn.2455-4529.IntJResDermatol20211708 Case Report Eosinophilic cellulitis (wells syndrome): a case report Ahmad Mohammad Baabdullah1, Khalid Ali Al Hawsawi2, Bashayr Saad Alhubayshi3*, Marwa Rashed Gammash4 1Department of Dermatology, King Abdulaziz University Hospital, Jeddah, Saudi Arabia 2 Department of Dermatology, King Faisal Specialist Hospital and Research Centre, Jeddah, Saudi Arabia 3Taibah College of Medicine, Taibah University, Almadinah Almunawwarah, Saudi Arabia 4Collage of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia Received: 14 January 2021 Accepted: 08 February 2021 *Correspondence: Dr. Bashayr Saad Alhubayshi, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Eosinophilic Cellulitis is also known as Wells syndrome is uncommon dermatitis, characterized by the infiltration of eosinophils in the dermis. The exact etiology of the disease is unknown. Clinically, it is highly varied but commonly the presentation is pruritic erythematous plaque. We report a case of one and half years old healthy boy who developed itchy bullae on the dorsum of his hand with multiple erythematous papules over his extremities that started immediately after his vaccines. Histopathological examination of the lesion showed infiltrate eosinophils with typical flame figures. The case was successfully treated with corticosteroid course. -

Case Report and Review of Wells' Syndrome in Childhood Amy E
Bullous “Cellulitis” With Eosinophilia: Case Report and Review of Wells’ Syndrome in Childhood Amy E. Gilliam, MD*; Anna L. Bruckner, MD‡; Rene´e M. Howard, MD*; Brian P. Lee, MD§; Susan Wu, MD§; and Ilona J. Frieden, MD* ABSTRACT. A 1-year-old girl presented with acute on- but later simplified the name to eosinophilic celluli- set of edematous erythematous plaques associated with tis.2 bullae on her extremities and accompanied by peripheral Wells’ syndrome is seen more commonly among eosinophilia. She was afebrile, and the skin lesions were adults but has been observed among children. Some pruritic but not tender. The patient was treated with hypothesize that this syndrome may represent a hy- intravenously administered antibiotics for presumed cel- persensitivity response to a circulating antigen.2 As- lulitis, without improvement. However, the lesions re- sponded rapidly to systemic steroid therapy. On the basis sociated precipitants include insect bites, medication of lesional morphologic features, peripheral eosino- reactions, recent immunization, myeloproliferative philia, and cutaneous histopathologic features, a diagno- disorders, malignancies, and infections. We describe sis of Wells’ syndrome was made. Wells’ syndrome is a case of a young child with no identifiable triggering extremely rare in childhood, with 27 pediatric cases re- factors, and we review the evidence for evaluation ported in the literature. Because it is seen so infre- and management of these pediatric cases. quently, there are no specific guidelines for evaluation and management of Wells’ syndrome among children. CASE REPORT The diagnosis should be considered for children with A previously healthy, 1-year-old girl presented with acute on- presumed cellulitis and eosinophilia who fail to respond set of edematous erythematous plaques, with associated bullae, on to antibiotics. -

Bullous Eosinophilic Annular Erythema
Volume 27 Number 5| May 2021 Dermatology Online Journal || Photo Vignette 27(5):16 Bullous eosinophilic annular erythema Yun Pei Koh1, Hong Liang Tey1-3 Affiliations: 1National Skin Centre, Singapore, 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 3Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore Corresponding Author: Koh Yun Pei, National Skin Centre, 1 Mandalay Road, Singapore 308405, Singapore, Tel: 65-2534455, Email: [email protected] areas of central clearing (Figure 1). Some plaques Abstract were studded with tense vesicles containing yellow Eosinophilic annular erythema is an idiopathic acute serous fluid, coalescing to form large bullae over his eosinophilic dermatosis. It is a rare condition, with left flank (Figure 2). Total body surface area involved approximately 30 cases reported in the English was 12%. literature. It features annular, figurate urticarial edematous plaques primarily affecting the trunk and Histological examination showed superficial and proximal limbs. During evaluation of a patient, deep perivascular infiltrate of predominantly secondary causes of eosinophilic inflammation such eosinophils and some lymphocytes (Figure 3). as allergy-related conditions (eczema, drug, urticaria, Epidermal involvement, apoptotic keratinocytes, contact dermatitis), parasitic infestations, and interface dermatitis, blistering, vasculitis, or autoimmune dermatoses will need to be excluded. granulomatous inflammation were all absent. Direct We present an unusual case of a 47-year-old patient who developed this condition. Keywords: eosinophilic dermatoses, urticaria Introduction Idiopathic primary eosinophilic dermatoses are a group of primarily eosinophil-driven skin disease characterized by moderate-to-dense eosinophilic skin infiltration with no significant infiltration of other leukocytes. Our case is consistent with one subtype of these conditions—eosinophilic annular erythema (EAE). -
PDF Full-Text
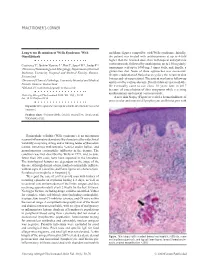
PRACTITIONER'S CORNER Long-term Remission of Wells Syndrome With and flame figures compatible with Wells syndrome. Initially, Omalizumab the patient was treated with antihistamines at up to 4-fold higher than the licensed dose, then with topical and systemic corticosteroids, followed by azathioprine up to 150 mg daily, Coattrenec Y1, Ibrahim Yasmine L2, Harr T1, Spoerl D1*, Jandus P1* tranexamic acid up to 1000 mg 3 times daily, and, finally, a 1Division of Immunology and Allergology, Department of Internal gluten-free diet. None of these approaches was successful. Medicine, University Hospital and Medical Faculty, Geneva, Despite eradication of Helicobacter pylori, the recurrent skin Switzerland lesions and edema persisted. The patient was lost to follow-up 2Division of Clinical Pathology, University Hospital and Medical and treated by various doctors. Detailed data are not available. Faculty, Geneva, Switzerland He eventually came to our clinic 10 years later in 2017 *DS and PJ contributed equally to this work. because of exacerbation of skin symptoms while receiving antihistamines and topical corticosteroids. J Investig Allergol Clin Immunol 2020; Vol. 30(1): 58-59 doi: 10.18176/jiaci.0436 A new skin biopsy (Figure) revealed a dermal infiltrate of perivascular and interstitial lymphocytes and histiocytes with Key words: Wells syndrome. Eosinophilic cellulitis. Omalizumab. Successful treatment. Palabras clave: Síndrome Wells. Celulitis eosinofílica. Omalizumab. Tratamiento eficaz. Eosinophilic cellulitis (Wells syndrome) is an uncommon recurrent inflammatory dermatosis. It is characterized by wide clinical variability comprising itching and/or burning tender erythematous lesions, sometimes with urticaria, vesicles and/or bullae, and granulomatous eosinophilic infiltrates in the dermis. The condition was first described by Wells in 1971, and, to date, fewer than 200 cases have been reported in the literature. -

March 7, 2014
ACP AMERICAN COLLEGE OF PHYSICIANS INTERNAL MEDICINE Doctors for Adults NEW JERSEY CHAPTER AMERICAN COLLEGE OF PHYSICIANS REGIONAL SCIENTIFIC MEETING ASSOCIATES ABSTRACT COMPETITION MARCH 7, 2014 PARTICIPATING INSTITUTIONS: Atlantic Health System - Morristown Memorial Hospital - Overlook Hospital Atlanticare Regional Medical Center Barnabas Health System - Monmouth Medical Center - Newark Beth Israel Medical Center - Saint Barnabas Medical Center Capital Health System Drexel University College of Medicine, Saint Peter’s University Hospital HUMC Mountainside Hospital Meridian Health System - Jersey Shore University Medical Center Mount Sinai School of Medicine - Englewood - Jersey City Medical Center Palisades Medical Center Raritan Bay Medical Center Rowan University - Cooper Medical School - School of Osteopathic Medicine Rutgers - Robert Wood Johnson Medical School - New Jersey Medical School Seton Hall University School of Health and Sciences Medical Science - Trinitas Regional Medical Center - St. Francis Medical Center COMMITTEE MEMBERS: William E. Farrer, MD, FACP – Chairperson Neil Kothari, MD, FACP Ed Liu, MD- Co- Chairperson Michael B. Steinberg, MD, MPH, FACP Vallur Thirumavalavan, MD Shuvendu Sen, MD DISCLAIMER: It is assumed that all participants adhered to the rules as stated in the original abstract submission form. It is also assumed that the abstracts submitted were original works, represented by the true authors. The abstracts appear in no particular order. Judging was performed in an attempt to minimize bias. Judges were unaware of the authors or institutions the competitor unless they were directly involved with the associate. Although there were many excellent abstracts those selected to be presented as poster or oral presentation were chosen on the basis of content. This content was felt to be intriguing from a clinical education standpoint, thought provoking, or could stimulate debate regarding our current practice of medicine. -

Update on Vasculitis: Overview and Relevant
An Bras Dermatol. 2020;95(4):493---507 Anais Brasileiros de Dermatologia www.anaisdedermatologia.org.br REVIEW Update on vasculitis: overview and relevant dermatological aspects for the clinical and ଝ,ଝଝ histopathological diagnosis --- Part II a,∗ b Thâmara Cristiane Alves Batista Morita , Paulo Ricardo Criado , b a a Roberta Fachini Jardim Criado , Gabriela Franco S. Trés , Mirian Nacagami Sotto a Department of Dermatology, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil b Dermatology Discipline, Faculdade de Medicina do ABC, Santo André, SP, Brazil Received 8 December 2019; accepted 28 April 2020 Available online 24 May 2020 Abstract Vasculitis is a group of several clinical conditions in which the main histopathological KEYWORDS finding is fibrinoid necrosis in the walls of blood vessels. This article assesses the main derma- Anti-neutrophil tological aspects relevant to the clinical and laboratory diagnosis of small- and medium-vessel cytoplasmic cutaneous and systemic vasculitis syndromes. The most important aspects of treatment are also antibodies; discussed. Churg-Strauss © 2020 Sociedade Brasileira de Dermatologia. Published by Elsevier Espana,˜ S.L.U. This is an syndrome; open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). Henoch-Schönlein purple; Leukocytoclastic cutaneous vasculitis; Systemic vasculitis; Vasculitis; Vasculitis associated with lupus of the central nervous system ଝ How to cite this article: Morita TCAB, Criado PR, Criado RFJ, Trés GFS, Sotto MN. Update on vasculitis: overview and relevant dermato- logical aspects for the clinical and histopathological diagnosis --- Part II. An Bras Dermatol. 2020;95:493---507. ଝଝ Study conducted at the Department of Dermatology, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil. -

INFECTIOUS DISEASES of HAITI Free
INFECTIOUS DISEASES OF HAITI Free. Promotional use only - not for resale. Infectious Diseases of Haiti - 2010 edition Infectious Diseases of Haiti - 2010 edition Copyright © 2010 by GIDEON Informatics, Inc. All rights reserved. Published by GIDEON Informatics, Inc, Los Angeles, California, USA. www.gideononline.com Cover design by GIDEON Informatics, Inc No part of this book may be reproduced or transmitted in any form or by any means without written permission from the publisher. Contact GIDEON Informatics at [email protected]. ISBN-13: 978-1-61755-090-4 ISBN-10: 1-61755-090-6 Visit http://www.gideononline.com/ebooks/ for the up to date list of GIDEON ebooks. DISCLAIMER: Publisher assumes no liability to patients with respect to the actions of physicians, health care facilities and other users, and is not responsible for any injury, death or damage resulting from the use, misuse or interpretation of information obtained through this book. Therapeutic options listed are limited to published studies and reviews. Therapy should not be undertaken without a thorough assessment of the indications, contraindications and side effects of any prospective drug or intervention. Furthermore, the data for the book are largely derived from incidence and prevalence statistics whose accuracy will vary widely for individual diseases and countries. Changes in endemicity, incidence, and drugs of choice may occur. The list of drugs, infectious diseases and even country names will vary with time. © 2010 GIDEON Informatics, Inc. www.gideononline.com All Rights Reserved. Page 2 of 314 Free. Promotional use only - not for resale. Infectious Diseases of Haiti - 2010 edition Introduction: The GIDEON e-book series Infectious Diseases of Haiti is one in a series of GIDEON ebooks which summarize the status of individual infectious diseases, in every country of the world. -

Eosinophils in Autoimmune Diseases
Eosinophils in Autoimmune Diseases The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Diny, Nicola L., Noel R. Rose, and Daniela Čiháková. 2017. “Eosinophils in Autoimmune Diseases.” Frontiers in Immunology 8 (1): 484. doi:10.3389/fimmu.2017.00484. http://dx.doi.org/10.3389/ fimmu.2017.00484. Published Version doi:10.3389/fimmu.2017.00484 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:33029710 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA REVIEW published: 27 April 2017 doi: 10.3389/fimmu.2017.00484 Eosinophils in Autoimmune Diseases Nicola L. Diny1, Noel R. Rose2 and Daniela Cˇiháková3* 1 W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA, 2 Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA, 3 Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Eosinophils are multifunctional granulocytes that contribute to initiation and modulation of inflammation. Their role in asthma and parasitic infections has long been recognized. Growing evidence now reveals a role for eosinophils in autoimmune diseases. In this review, we summarize the function of eosinophils in inflammatory bowel diseases, neu- romyelitis optica, bullous pemphigoid, autoimmune myocarditis, primary biliary cirrhosis, eosinophilic granulomatosis with polyangiitis, and other autoimmune diseases. -

Wells' Syndrome Proceeded by Leukocytoclastic Vasculitis
Wells’ Syndrome Proceeded by Leukocytoclastic Vasculitis: A Discussion with New Insights into Analogous Pathophysiology S. Brandon Nickle, DO,* Roxanne Rajaii, MS, DO,** Zachary Hopkins, BS,*** Nelson Charlie, MD**** *Dermatology Resident , 4th year, Broward Health Medical Center, Ft. Lauderdale, FL **Dermatology Resident, 2nd year, Beaumont Hospital, Farmington Hills, MI ***Medical Student, 4th year, University of Utah School of Medicine, Salt Lake City, UT ****Attending Dermatologist, Lauderdale Academic Dermatology at Broward Medical Center, Ft. Lauderdale, FL Disclosures: None Correspondence: Brandon Nickle, DO; 4331 SW 78th Dr., Davie, FL 33328; Fax: 801-318-1431; [email protected] Abstract Wells’ syndrome, also referred to as eosinophilic cellulitis, is a rare inflammatory dermatosis. Although its etiology is unknown, it is thought to be an abnormal eosinophilic response to one of various causative agents. Its cutaneous manifestations vary in morphology and severity, but the disease often follows a relapsing-remitting course. We report the case of a patient with Wells’ syndrome preceded by leukocytoclastic vasculitis (LCV). Introduction bulla on the left lateral leg, and one perilesional syndrome. The patient was given a one-month Wells’ syndrome, also referred to as eosinophilic biopsy from skin directly adjacent to the bulla. course of prednisone and was free of symptoms cellulitis, is an uncommon inflammatory They were sent for direct immunofluorescence at three-month follow-up. She was lost to dermatosis, with fewer than 200 cases reported (DIF), which revealed an overwhelming number further follow-up. worldwide.2 It was first described in 1971 by of eosinophils in the superficial and deep dermis George Wells, noted as a rare entity with an (Figures 5, 6 [p. -

UC Davis Dermatology Online Journal
UC Davis Dermatology Online Journal Title Successful treatment of eosinophilic cellulitis with dapsone Permalink https://escholarship.org/uc/item/9v67b10b Journal Dermatology Online Journal, 22(7) Authors Coelho de Sousa, Virgínia Laureano Oliveira, André Cardoso, Jorge Publication Date 2016 DOI 10.5070/D3227031648 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 22 Number 7 July 2016 Case Presentation Successful treatment of eosinophilic cellulitis with dapsone. Virgínia Coelho de Sousa, André Laureano Oliveira, Jorge Cardoso Dermatology Online Journal 22 (7): 11 Department of Dermatology and Venereology, Hospital de Curry Cabral – Centro Hospitalar de Lisboa Central, Lisboa, Portugal Correspondence: Virgínia Coelho de Sousa, MD Hospital de Curry Cabral – Centro Hospitalar de Lisboa Central, Lisboa, Portugal Rua da Beneficência, nº8, 1069-166 Lisboa, Portugal Tel. +351926826029 Email: [email protected] Abstract A 55-year-old woman presented with a 3-year history of recurrent episodes of pruritic cellulitis-like erythematous plaques, mostly located on the limbs. Simultaneously, fever, malaise and peripheral eosinophilia were noted. The clinical diagnosis of eosinophilic cellulitis (also known as Well’s syndrome) was supported by the histopathological finding of typical “flame figures”. Treatment with dapsone was initiated at a dose of 50 mg per day. After one year of follow-up the patient was relapse- free. Eosinophilic cellulitis is an uncommon, recurrent inflammatory skin disease. The management is often a challenge, due to the frequent need for long-term therapy. Dapsone is an effective and safe treatment option. Keywords: Dapsone, Eosinophilic cellulitis, Urticaria, Wells’ syndrome Introduction Eosinophilic cellulitis or Wells’ syndrome is a rare, recurrent inflammatory skin disease of unknown etiology [1]. -

Pediatric Allergy/Immunology/Rheumatology
Pediatric Allergy/Immunology/Rheumatology Akaluck Thatayatikom, MD Associate Professor Director, Division of Allergy/Immunology/Rheumatology Department of Pediatrics, University of Kentucky Disclosure: None Objectives Upon completion of this session, participants should be able t o und erst and , recogni ze and manage th e following conditions: • Common allergic diseases in children – Allergic Rhinoconjunctivitis – Atopic dermatitis – Food allergy • Common primary Immunodeficiency • Common rheumatologic diseases in children – Acute arthritis: ARF, reactive arthritis, Transient toxic synovitis, – Chronic arthritis: JRA (JIA) Allergic Diseases Asthma Reactions to foods Rhinitis Eczema/ Contact dermatitis Allergic Mechanisms Urticaria Reactions to Drugs Angioedema Anaphylaxis Allergy and allergic diseases 2nd edition Atopy and Atopic Diseases • Atopy: A genetically predisposed diathesis manifesting as exaggeratdted responses (eg. bronc hocons tititriction, I IEgE production, vasodilation, pruritus) to a variety of environmental stimuli (irritants, allergens, and mib)icrobes) • Atopy is fundamental to the pathogenesis of atopic allergic diseases; allergic rhinoconjunctivitis, asthma, food allergy, atopic dermatitis. • Not every atopic child develops atopic diseases • Not everyyp child with atopic disease is ato py. Atopy and Atopic Diseases • Objective evidence of being atopy: – Elevated total IgE in serum for age – Specific IgE to specific allergens: • In vivo: Specific IgE on mast cells (skin) – Prick skin test – Intradermal skin test • -

5 and IL-18 in Eosinophil Mediated Pathogenesis of Allergic Diseases T ⁎ Hemanth Kumar Kandikattu, Sathisha Upparahalli Venkateshaiah, Anil Mishra
Cytokine and Growth Factor Reviews 47 (2019) 83–98 Contents lists available at ScienceDirect Cytokine and Growth Factor Reviews journal homepage: www.elsevier.com/locate/cytogfr Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases T ⁎ Hemanth Kumar Kandikattu, Sathisha Upparahalli Venkateshaiah, Anil Mishra Department of Medicine, Tulane Eosinophilic Disorders Centre (TEDC), Section of Pulmonary Diseases, Tulane University School of Medicine, New Orleans, LA 70112, United States ARTICLE INFO ABSTRACT Keywords: Eosinophils are circulating granulocytes that have pleiotropic effects in response to inflammatory signals in the Allergy body. In response to allergens or pathogens, exposure eosinophils are recruited in various organs that execute Eosinophils pathological immune responses. IL-5 plays a key role in the differentiation, development, and survival of eo- IL-5 sinophils. Eosinophils are involved in a variety of allergic diseases including asthma, dermatitis and various IL-18 gastrointestinal disorders (EGID). IL-5 signal transduction involves JAK-STAT-p38MAPK-NFκB activation and Immune responses executes extracellular matrix remodeling, EMT transition and immune responses in allergic diseases. IL-18 is a Th2 responses classical cytokine also involved in immune responses and has a critical role in inflammasome pathway. We recently identified the IL-18 role in the generation, transformation, and maturation of (CD101+CD274+) pa- thogenic eosinophils. In, addition, several other cytokines like IL-2, IL-4, IL-13, IL-21, and IL-33 also contribute in advancing eosinophils associated immune responses in innate and adaptive immunity. This review discusses with a major focus (1) Eosinophils and its constituents, (2) Role of IL-5 and IL-18 in eosinophils development, transformation, maturation, signal transduction of IL-5 and IL-18, (3) The role of eosinophils in allergic disorders and (4) The role of several other associated cytokines in promoting eosinophils mediated allergic diseases.