Eosinophils in Autoimmune Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinic Offers a Comprehensive Approach to Immune-Mediated Digestive Diseases
Clinic offers a comprehensive approach to immune-mediated digestive diseases Treating a wide range of disorders “Our team designed this clinic to treat a wide range of allergic and immune-mediated gastrointestinal diseases,” says Laura Wozniak, MD, MS, co-director of the UCLA Pediatric Celiac Disease & Eosinophilic Esophagitis Clinic and assistant clinical professor of pediatric gastroenterology. “These conditions can be quite variable in terms of symptoms and management, which makes it important to involve an experienced, multidisciplinary team. UCLA is one of only a few centers across the country with a dedicated team to do just that.” The Pediatric Celiac Disease & Through collaboration among Pediatric Gastroenterology, Allergy & Immunology, Eosinophilic Esophagitis Clinic and Nutrition, the new Pediatric Celiac Disease & Eosinophilic Esophagitis Clinic at Mattel Children’s Hospital at Mattel Children’s Hospital UCLA offers comprehensive patient-centered care for UCLA sees patients with a wide children across a wide range of immune-mediated digestive diseases. It is the only range of disorders including: multidisciplinary clinic of its kind in Southern California. • Celiac disease and gluten sensitivity Immune-mediated digestive diseases • Eosinophilic esophagitis Celiac disease (CD) and eosinophilic esophagitis (EoE) are two of the more common and gastroenteritis allergic and immune-mediated digestive diseases among a diverse group of conditions • Food allergy and food sensitivity that affect the gastrointestinal (GI) tract. These disorders, which often develop in early • Food protein-induced childhood and require a lifetime of vigilant management, have been on the rise over enterocolitis syndrome (FPIES) the past decade although the underlying pathogenesis remains poorly understood. • Food protein intolerance (e.g., Symptoms can be nonspecific, and a lack of targeted testing and clinical biomarkers cow’s milk protein intolerance) cause the disorders to often go unrecognized or misdiagnosed. -

Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders During the SARS-Cov-2 Outbreak
Journal of Clinical Medicine Article Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders during the SARS-CoV-2 Outbreak Edoardo Vincenzo Savarino 1,* , Paola Iovino 2 , Antonella Santonicola 2 , Matteo Ghisa 1 , Giorgio Laserra 1, Brigida Barberio 1, Daria Maniero 1, Greta Lorenzon 1, Carolina Ciacci 2 , Vincenzo Savarino 3 and Fabiana Zingone 1 1 Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padua, 35128 Padua, Italy; [email protected] (M.G.); [email protected] (G.L.); [email protected] (B.B.); [email protected] (D.M.); [email protected] (G.L.); [email protected] (F.Z.) 2 Department of Medicine, Surgery, Dentistry, Scuola Medica Salernitana, University of Salerno, University Hospital San Giovanni di Dio e Ruggi d’Aragona, 84131 Salerno, Italy; [email protected] (P.I.); [email protected] (A.S.); [email protected] (C.C.) 3 Gastroenterology Unit, Department of Internal Medicine, University of Genoa, 16126 Genoa, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-049-8217749 Received: 4 June 2020; Accepted: 23 June 2020; Published: 26 June 2020 Abstract: Eosinophilic gastrointestinal diseases (EGIDs) are chronic gastrointestinal conditions requiring corticosteroid and immunosuppressive therapy for disease control. Patients with EGIDs usually report impaired quality of life. We aimed to report the clinical and psychological impact of COVID-19 infection in EGID patients. In this prospective web-based study we invited all consecutive EGID patients attending the University Hospital of Salerno (Campania) and Padua (Veneto) to fill an ad hoc COVID-19 survey. Moreover, a telemedicine service for direct consultation was organized. -

Eosinophilic Cellulitis (Wells Syndrome): a Case Report
International Journal of Research in Dermatology Baabdullah AM et al. Int J Res Dermatol. 2021 May;7(3):450-453 http://www.ijord.com DOI: https://dx.doi.org/10.18203/issn.2455-4529.IntJResDermatol20211708 Case Report Eosinophilic cellulitis (wells syndrome): a case report Ahmad Mohammad Baabdullah1, Khalid Ali Al Hawsawi2, Bashayr Saad Alhubayshi3*, Marwa Rashed Gammash4 1Department of Dermatology, King Abdulaziz University Hospital, Jeddah, Saudi Arabia 2 Department of Dermatology, King Faisal Specialist Hospital and Research Centre, Jeddah, Saudi Arabia 3Taibah College of Medicine, Taibah University, Almadinah Almunawwarah, Saudi Arabia 4Collage of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia Received: 14 January 2021 Accepted: 08 February 2021 *Correspondence: Dr. Bashayr Saad Alhubayshi, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Eosinophilic Cellulitis is also known as Wells syndrome is uncommon dermatitis, characterized by the infiltration of eosinophils in the dermis. The exact etiology of the disease is unknown. Clinically, it is highly varied but commonly the presentation is pruritic erythematous plaque. We report a case of one and half years old healthy boy who developed itchy bullae on the dorsum of his hand with multiple erythematous papules over his extremities that started immediately after his vaccines. Histopathological examination of the lesion showed infiltrate eosinophils with typical flame figures. The case was successfully treated with corticosteroid course. -

Hiatal Hernias in Patients with GERD-Like Symptoms: Evaluation of Dynamic Real-Time MRI Vs Endoscopy
European Radiology (2019) 29:6653–6661 https://doi.org/10.1007/s00330-019-06284-8 MAGNETIC RESONANCE Hiatal hernias in patients with GERD-like symptoms: evaluation of dynamic real-time MRI vs endoscopy Ali Seif Amir Hosseini1 & Johannes Uhlig1 & Ulrike Streit1 & Annemarie Uhlig2 & Thilo Sprenger3 & Edris Wedi4 & Volker Ellenrieder4 & Michael Ghadimi3 & Martin Uecker1,5 & Dirk Voit6 & Jens Frahm5,6 & Joachim Lotz1,5 & Lorenz Biggemann1 Received: 4 March 2019 /Revised: 17 April 2019 /Accepted: 24 May 2019 /Published online: 11June 2019 # European Society of Radiology 2019 Abstract Purpose To assess the diagnostic potential of real-time MRI for assessment of hiatal hernias in patients with GERD-like symptoms compared to endoscopy. Material and methods One hundred eight patients with GERD-like symptoms were included in this observational cohort study between 2015 and 2017. Real-time MRI was performed at 3.0 Tesla with temporal resolution of 40 ms, dynamically visualizing the esophageal transport of a pineapple juice bolus, its passage through the gastroesophageal junction, and functional responses during Valsalva maneuver. Hernia detection on MRI and endoscopy was calculated using contingency tables with diagnosis of hernia on either modality as reference. Results Of 108 patients, 107 underwent successful MRI without adverse events; 1 examination was aborted to inability to swallow pineapple juice in supine position. No perforation or acute bleeding occurred during endoscopy. Median examination time was 15 min. Eighty-five patients (79.4%) were diagnosed with hiatal hernia on either real-time MRI or endoscopy. Forty-six hernias were visible on both modalities. Seventeen hernias were evident exclusively on MRI, and 22 exclusively on endoscopy. -

Esophagitis Dissecans Associated with Eosinophilic Esophagitis in an Adolescent
Esophagitis dissecans associated with eosinophilic esophagitis in an adolescent Marjorie-Anne R. Guerra 1*, Elaheh Vahabnezhad 2, Eric Swanson 3, Bita V. Naini 3, Laura J. Wozniak 2 1 Department of Pediatrics, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA 2 Pediatric Gastroenterology, Hepatology, and Nutrition, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA 3 Department of Pathology, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA Abstract Esophagitis dissecans superficialis and eosinophilic esophagitis are distinct esophageal pathologies with characteristic clinical and histologic findings. Esophagitis dissecans superficialis is a rare finding on endoscopy consisting of the peeling of large fragments of esophageal mucosa. Histology shows sloughing of the epithelium and parakeratosis. Eosinophilic esophagitis is an allergic disease of the esophagus characterized by eosinophilic inflammation of the epithelium and symptoms of esophageal dysfunction. Both of these esophageal processes have been associated with other diseases, but there is no known association between them. We describe a case of esophagitis dissecans superficialis and eosinophilic esophagitis in an adolescent patient. To our knowledge, this is the first case describing an association between esophageal dissecans superficialis and eosinophilic esophagitis. Citation: Guerra MR, Vahabnezhad E, Swanson E, Naini BV, Wozniak LJ (2015) Esophagitis dissecans associated with eosinophilic esophagitis in an adolescent. Adv Pediatr Res 2:8. doi:10.12715/apr.2015.2.8 Received: January 27, 2015; Accepted: February 19, 2015; Published: March 19, 2015 Copyright: © 2015 Guerra et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Eosinophilic Ascites and Duodenal Obstruction in a Patient with Liver Cirrhosis
Hindawi Publishing Corporation Case Reports in Gastrointestinal Medicine Volume 2014, Article ID 928496, 4 pages http://dx.doi.org/10.1155/2014/928496 Case Report Eosinophilic Ascites and Duodenal Obstruction in a Patient with Liver Cirrhosis Nasrollah Maleki,1 Mohammadreza Kalantar Hormozi,2 Mehrzad Bahtouee,3 Zahra Tavosi,3 Hamidreza Mosallai Pour,3 and Seiiedeh Samaneh Taghiyan Jamaleddin Kolaii3 1 Department of Internal Medicine, Imam Khomeini Hospital, Ardabil University of Medical Sciences, Ardabil, Iran 2 The Persian Gulf Marine Medicine Biotechnology Research Center, Department of Endocrinology, Bushehr University of Medical Sciences, Bushehr, Iran 3 Department of Internal Medicine, Shohadaye Khalije Fars Hospital, Bushehr University of Medical Sciences, Bushehr, Iran Correspondence should be addressed to Nasrollah Maleki; [email protected] Received 2 December 2013; Accepted 1 January 2014; Published 10 February 2014 Academic Editors: H. Akiho, G. Bassotti, O. I. Giouleme, and I. M. Leitman Copyright © 2014 Nasrollah Maleki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Eosinophilic gastroenteritis (EG) is a rare disease characterized by eosinophilic infiltration of portions of the gastrointestinal tract. EosinophilicascitesisprobablythemostunusualandrarepresentationofEGandisgenerallyassociatedwiththeserosalform of EG. Hereby, we report a case of eosinophilic ascites with duodenal obstruction in a patient with liver cirrhosis. A 50-year-old woman was admitted to our hospital because of abdominal pain, nausea, bloating, and constipation. She had a history of laparotomy because of duodenal obstruction 2 years ago. Based on clinical, radiological, endoscopic, and pathological findings, and given the excluding the other causes of peripheral eosinophilia, the diagnosis of eosinophilic gastroenteritis along with liver cirrhosis and spontaneous bacterial peritonitis was established. -

Case Report and Review of Wells' Syndrome in Childhood Amy E
Bullous “Cellulitis” With Eosinophilia: Case Report and Review of Wells’ Syndrome in Childhood Amy E. Gilliam, MD*; Anna L. Bruckner, MD‡; Rene´e M. Howard, MD*; Brian P. Lee, MD§; Susan Wu, MD§; and Ilona J. Frieden, MD* ABSTRACT. A 1-year-old girl presented with acute on- but later simplified the name to eosinophilic celluli- set of edematous erythematous plaques associated with tis.2 bullae on her extremities and accompanied by peripheral Wells’ syndrome is seen more commonly among eosinophilia. She was afebrile, and the skin lesions were adults but has been observed among children. Some pruritic but not tender. The patient was treated with hypothesize that this syndrome may represent a hy- intravenously administered antibiotics for presumed cel- persensitivity response to a circulating antigen.2 As- lulitis, without improvement. However, the lesions re- sponded rapidly to systemic steroid therapy. On the basis sociated precipitants include insect bites, medication of lesional morphologic features, peripheral eosino- reactions, recent immunization, myeloproliferative philia, and cutaneous histopathologic features, a diagno- disorders, malignancies, and infections. We describe sis of Wells’ syndrome was made. Wells’ syndrome is a case of a young child with no identifiable triggering extremely rare in childhood, with 27 pediatric cases re- factors, and we review the evidence for evaluation ported in the literature. Because it is seen so infre- and management of these pediatric cases. quently, there are no specific guidelines for evaluation and management of Wells’ syndrome among children. CASE REPORT The diagnosis should be considered for children with A previously healthy, 1-year-old girl presented with acute on- presumed cellulitis and eosinophilia who fail to respond set of edematous erythematous plaques, with associated bullae, on to antibiotics. -

Bullous Eosinophilic Annular Erythema
Volume 27 Number 5| May 2021 Dermatology Online Journal || Photo Vignette 27(5):16 Bullous eosinophilic annular erythema Yun Pei Koh1, Hong Liang Tey1-3 Affiliations: 1National Skin Centre, Singapore, 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 3Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore Corresponding Author: Koh Yun Pei, National Skin Centre, 1 Mandalay Road, Singapore 308405, Singapore, Tel: 65-2534455, Email: [email protected] areas of central clearing (Figure 1). Some plaques Abstract were studded with tense vesicles containing yellow Eosinophilic annular erythema is an idiopathic acute serous fluid, coalescing to form large bullae over his eosinophilic dermatosis. It is a rare condition, with left flank (Figure 2). Total body surface area involved approximately 30 cases reported in the English was 12%. literature. It features annular, figurate urticarial edematous plaques primarily affecting the trunk and Histological examination showed superficial and proximal limbs. During evaluation of a patient, deep perivascular infiltrate of predominantly secondary causes of eosinophilic inflammation such eosinophils and some lymphocytes (Figure 3). as allergy-related conditions (eczema, drug, urticaria, Epidermal involvement, apoptotic keratinocytes, contact dermatitis), parasitic infestations, and interface dermatitis, blistering, vasculitis, or autoimmune dermatoses will need to be excluded. granulomatous inflammation were all absent. Direct We present an unusual case of a 47-year-old patient who developed this condition. Keywords: eosinophilic dermatoses, urticaria Introduction Idiopathic primary eosinophilic dermatoses are a group of primarily eosinophil-driven skin disease characterized by moderate-to-dense eosinophilic skin infiltration with no significant infiltration of other leukocytes. Our case is consistent with one subtype of these conditions—eosinophilic annular erythema (EAE). -
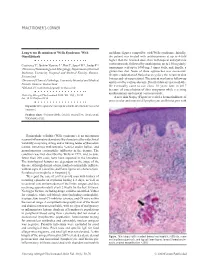
PDF Full-Text
PRACTITIONER'S CORNER Long-term Remission of Wells Syndrome With and flame figures compatible with Wells syndrome. Initially, Omalizumab the patient was treated with antihistamines at up to 4-fold higher than the licensed dose, then with topical and systemic corticosteroids, followed by azathioprine up to 150 mg daily, Coattrenec Y1, Ibrahim Yasmine L2, Harr T1, Spoerl D1*, Jandus P1* tranexamic acid up to 1000 mg 3 times daily, and, finally, a 1Division of Immunology and Allergology, Department of Internal gluten-free diet. None of these approaches was successful. Medicine, University Hospital and Medical Faculty, Geneva, Despite eradication of Helicobacter pylori, the recurrent skin Switzerland lesions and edema persisted. The patient was lost to follow-up 2Division of Clinical Pathology, University Hospital and Medical and treated by various doctors. Detailed data are not available. Faculty, Geneva, Switzerland He eventually came to our clinic 10 years later in 2017 *DS and PJ contributed equally to this work. because of exacerbation of skin symptoms while receiving antihistamines and topical corticosteroids. J Investig Allergol Clin Immunol 2020; Vol. 30(1): 58-59 doi: 10.18176/jiaci.0436 A new skin biopsy (Figure) revealed a dermal infiltrate of perivascular and interstitial lymphocytes and histiocytes with Key words: Wells syndrome. Eosinophilic cellulitis. Omalizumab. Successful treatment. Palabras clave: Síndrome Wells. Celulitis eosinofílica. Omalizumab. Tratamiento eficaz. Eosinophilic cellulitis (Wells syndrome) is an uncommon recurrent inflammatory dermatosis. It is characterized by wide clinical variability comprising itching and/or burning tender erythematous lesions, sometimes with urticaria, vesicles and/or bullae, and granulomatous eosinophilic infiltrates in the dermis. The condition was first described by Wells in 1971, and, to date, fewer than 200 cases have been reported in the literature. -

March 7, 2014
ACP AMERICAN COLLEGE OF PHYSICIANS INTERNAL MEDICINE Doctors for Adults NEW JERSEY CHAPTER AMERICAN COLLEGE OF PHYSICIANS REGIONAL SCIENTIFIC MEETING ASSOCIATES ABSTRACT COMPETITION MARCH 7, 2014 PARTICIPATING INSTITUTIONS: Atlantic Health System - Morristown Memorial Hospital - Overlook Hospital Atlanticare Regional Medical Center Barnabas Health System - Monmouth Medical Center - Newark Beth Israel Medical Center - Saint Barnabas Medical Center Capital Health System Drexel University College of Medicine, Saint Peter’s University Hospital HUMC Mountainside Hospital Meridian Health System - Jersey Shore University Medical Center Mount Sinai School of Medicine - Englewood - Jersey City Medical Center Palisades Medical Center Raritan Bay Medical Center Rowan University - Cooper Medical School - School of Osteopathic Medicine Rutgers - Robert Wood Johnson Medical School - New Jersey Medical School Seton Hall University School of Health and Sciences Medical Science - Trinitas Regional Medical Center - St. Francis Medical Center COMMITTEE MEMBERS: William E. Farrer, MD, FACP – Chairperson Neil Kothari, MD, FACP Ed Liu, MD- Co- Chairperson Michael B. Steinberg, MD, MPH, FACP Vallur Thirumavalavan, MD Shuvendu Sen, MD DISCLAIMER: It is assumed that all participants adhered to the rules as stated in the original abstract submission form. It is also assumed that the abstracts submitted were original works, represented by the true authors. The abstracts appear in no particular order. Judging was performed in an attempt to minimize bias. Judges were unaware of the authors or institutions the competitor unless they were directly involved with the associate. Although there were many excellent abstracts those selected to be presented as poster or oral presentation were chosen on the basis of content. This content was felt to be intriguing from a clinical education standpoint, thought provoking, or could stimulate debate regarding our current practice of medicine. -

Elemental Diet Is an Effective Treatment for Eosinophilic Esophagitis in Children and Adolescents Jonathan E
THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol. 98, No. 4, 2003 © 2003 by Am. Coll. of Gastroenterology ISSN 0002-9270/03/$30.00 Published by Elsevier Science Inc. doi:10.1016/S0002-9270(03)00054-6 Elemental Diet Is an Effective Treatment for Eosinophilic Esophagitis in Children and Adolescents Jonathan E. Markowitz, M.D., Jonathan M. Spergel, M.D., Ph.D., Eduardo Ruchelli, M.D., and Chris A. Liacouras, M.D. Divisions of Gastroenterology and Nutrition, and Allergy, Immunology, and Infectious Diseases, and Department of Pathology, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania OBJECTIVE: Eosinophilic esophagitis (EoE), a disorder char- troesophageal reflux disease (GERD) and include vomiting, acterized by eosinophilic infiltration of the esophageal mu- regurgitation, nausea, epigastric pain, heartburn, and dys- cosa, has been defined in large part through published case phagia. In both groups, the symptoms typically improve reports and series leading to ambiguity in both diagnostic with acid blockade; however, whereas patients with GERD and treatment options. Corticosteroids, cromolyn, and ele- generally become symptom free and demonstrate a resolu- mental diet have all been reported as successful treatments tion in their esophagitis, children with EoE almost always for EoE. In this study, we sought to accurately define a continue to exhibit clinical symptoms and display no histo- population of patients with EoE and then assess their re- logic improvement despite aggressive acid blockade. sponse to elemental diet. The spectrum of EoE has been described predominantly by case reports and case series. However, in the majority of METHODS: A series of patients with chronic symptoms of these reports, there has been considerable variability in the gastroesophageal reflux disease and an isolated esophageal criteria used to define EoE. -

Update on Vasculitis: Overview and Relevant
An Bras Dermatol. 2020;95(4):493---507 Anais Brasileiros de Dermatologia www.anaisdedermatologia.org.br REVIEW Update on vasculitis: overview and relevant dermatological aspects for the clinical and ଝ,ଝଝ histopathological diagnosis --- Part II a,∗ b Thâmara Cristiane Alves Batista Morita , Paulo Ricardo Criado , b a a Roberta Fachini Jardim Criado , Gabriela Franco S. Trés , Mirian Nacagami Sotto a Department of Dermatology, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil b Dermatology Discipline, Faculdade de Medicina do ABC, Santo André, SP, Brazil Received 8 December 2019; accepted 28 April 2020 Available online 24 May 2020 Abstract Vasculitis is a group of several clinical conditions in which the main histopathological KEYWORDS finding is fibrinoid necrosis in the walls of blood vessels. This article assesses the main derma- Anti-neutrophil tological aspects relevant to the clinical and laboratory diagnosis of small- and medium-vessel cytoplasmic cutaneous and systemic vasculitis syndromes. The most important aspects of treatment are also antibodies; discussed. Churg-Strauss © 2020 Sociedade Brasileira de Dermatologia. Published by Elsevier Espana,˜ S.L.U. This is an syndrome; open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). Henoch-Schönlein purple; Leukocytoclastic cutaneous vasculitis; Systemic vasculitis; Vasculitis; Vasculitis associated with lupus of the central nervous system ଝ How to cite this article: Morita TCAB, Criado PR, Criado RFJ, Trés GFS, Sotto MN. Update on vasculitis: overview and relevant dermato- logical aspects for the clinical and histopathological diagnosis --- Part II. An Bras Dermatol. 2020;95:493---507. ଝଝ Study conducted at the Department of Dermatology, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.