Update on Vasculitis: Overview and Relevant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of Vasculitis Joseph L
Classification of Vasculitis Joseph L. Jorizzo A working classification of necrotizing vasculitis based on size of the affected vessel is proposed. The classification proposed by Gilliam and Fink in 1976 is a basis for the curren proposal. A revised working classification of vasculitis is presented. Small vessel necrotizing vasculitis and larger vessel necrotizing vasculitis categories are further subdivided. Improved understanding of the basic science aspects of vasculitis will hopefully give rise to a better consensus on the classification of vasculitis. J Invest Dermatol 100:106S–110S, 1993 A considerable portion of the now classic Medical Grand Rounds on SMALL VESSEL NECROTIZING VASCULITIS necrotizing vasculitis presented at The University of Texas Southwestern (NECROTIZING VENULITIS) Medical Center by James W. Gilliam in 1976 dealt with the classification of necrotizing vasculitis. Aspects of this presentation have been The hallmark of small-vessel necrotizing vasculitis is the diagnostic published (Table I) [1]. histopathologic finding of leukocytoclastic vasculitis including endothe- The historical context of Gilliam’s classification is important given the lial cell swelling, neutrophilic invasion of blood-vessel walls, leukocy- confounding array of classification schemas that preceded (and followed!) toclasia (neutrophilic nuclear karyorrhexis), extravasation of erythrocytes, and fibrinoid necrosis of blood vessel walls [14]. This it. The classification of vasculitis remains frustratingly controversial today. Zeek was the first to incorporate a clinicopathologic assessment process has been shown to affect post capillary venules [15,16]. This based on the size of the vessel involved in the inflammatory process in entity has been proposed as a cutaneous model for circulating immune his classification of necrotizing vasculitis in 1952 [2]. -

Visual Recognition of Autoimmune Connective Tissue Diseases
Seeing the Signs: Visual Recognition of Autoimmune Connective Tissue Diseases Utah Association of Family Practitioners CME Meeting at Snowbird, UT 1:00-1:30 pm, Saturday, February 13, 2016 Snowbird/Alta Rick Sontheimer, M.D. Professor of Dermatology Univ. of Utah School of Medicine Potential Conflicts of Interest 2016 • Consultant • Paid speaker – Centocor (Remicade- – Winthrop (Sanofi) infliximab) • Plaquenil – Genentech (Raptiva- (hydroxychloroquine) efalizumab) – Amgen (etanercept-Enbrel) – Alexion (eculizumab) – Connetics/Stiefel – MediQuest • Royalties Therapeutics – Lippincott, – P&G (ChelaDerm) Williams – Celgene* & Wilkins* – Sanofi/Biogen* – Clearview Health* Partners • 3Gen – Research partner *Active within past 5 years Learning Objectives • Compare and contrast the presenting and Hallmark cutaneous manifestations of lupus erythematosus and dermatomyositis • Compare and contrast the presenting and Hallmark cutaneous manifestations of morphea and systemic sclerosis Distinguishing the Cutaneous Manifestations of LE and DM Skin involvement is 2nd most prevalent clinical manifestation of SLE and 2nd most common presenting clinical manifestation Comprehensive List of Skin Lesions Associated with LE LE-SPECIFIC LE-NONSPECIFIC Cutaneous vascular disease Acute Cutaneous LE Vasculitis Leukocytoclastic Localized ACLE Palpable purpura Urticarial vasculitis Generalized ACLE Periarteritis nodosa-like Ten-like ACLE Vasculopathy Dego's disease-like Subacute Cutaneous LE Atrophy blanche-like Periungual telangiectasia Annular Livedo reticularis -

Review Cutaneous Patterns Are Often the Only Clue to a a R T I C L E Complex Underlying Vascular Pathology
pp11 - 46 ABstract Review Cutaneous patterns are often the only clue to a A R T I C L E complex underlying vascular pathology. Reticulate pattern is probably one of the most important DERMATOLOGICAL dermatological signs of venous or arterial pathology involving the cutaneous microvasculature and its MANIFESTATIONS OF VENOUS presence may be the only sign of an important underlying pathology. Vascular malformations such DISEASE. PART II: Reticulate as cutis marmorata congenita telangiectasia, benign forms of livedo reticularis, and sinister conditions eruptions such as Sneddon’s syndrome can all present with a reticulate eruption. The literature dealing with this KUROSH PARSI MBBS, MSc (Med), FACP, FACD subject is confusing and full of inaccuracies. Terms Departments of Dermatology, St. Vincent’s Hospital & such as livedo reticularis, livedo racemosa, cutis Sydney Children’s Hospital, Sydney, Australia marmorata and retiform purpura have all been used to describe the same or entirely different conditions. To our knowledge, there are no published systematic reviews of reticulate eruptions in the medical Introduction literature. he reticulate pattern is probably one of the most This article is the second in a series of papers important dermatological signs that signifies the describing the dermatological manifestations of involvement of the underlying vascular networks venous disease. Given the wide scope of phlebology T and its overlap with many other specialties, this review and the cutaneous vasculature. It is seen in benign forms was divided into multiple instalments. We dedicated of livedo reticularis and in more sinister conditions such this instalment to demystifying the reticulate as Sneddon’s syndrome. There is considerable confusion pattern. -

Eosinophilic Cellulitis (Wells Syndrome): a Case Report
International Journal of Research in Dermatology Baabdullah AM et al. Int J Res Dermatol. 2021 May;7(3):450-453 http://www.ijord.com DOI: https://dx.doi.org/10.18203/issn.2455-4529.IntJResDermatol20211708 Case Report Eosinophilic cellulitis (wells syndrome): a case report Ahmad Mohammad Baabdullah1, Khalid Ali Al Hawsawi2, Bashayr Saad Alhubayshi3*, Marwa Rashed Gammash4 1Department of Dermatology, King Abdulaziz University Hospital, Jeddah, Saudi Arabia 2 Department of Dermatology, King Faisal Specialist Hospital and Research Centre, Jeddah, Saudi Arabia 3Taibah College of Medicine, Taibah University, Almadinah Almunawwarah, Saudi Arabia 4Collage of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia Received: 14 January 2021 Accepted: 08 February 2021 *Correspondence: Dr. Bashayr Saad Alhubayshi, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Eosinophilic Cellulitis is also known as Wells syndrome is uncommon dermatitis, characterized by the infiltration of eosinophils in the dermis. The exact etiology of the disease is unknown. Clinically, it is highly varied but commonly the presentation is pruritic erythematous plaque. We report a case of one and half years old healthy boy who developed itchy bullae on the dorsum of his hand with multiple erythematous papules over his extremities that started immediately after his vaccines. Histopathological examination of the lesion showed infiltrate eosinophils with typical flame figures. The case was successfully treated with corticosteroid course. -

Update on Vasculitis: Overview and Relevant
An Bras Dermatol. 2020;95(4):493---507 Anais Brasileiros de Dermatologia www.anaisdedermatologia.org.br REVIEW Update on vasculitis: overview and relevant dermatological aspects for the clinical and ଝ,ଝଝ histopathological diagnosis --- Part II a,∗ b Thâmara Cristiane Alves Batista Morita , Paulo Ricardo Criado , b a a Roberta Fachini Jardim Criado , Gabriela Franco S. Trés , Mirian Nacagami Sotto a Department of Dermatology, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil b Dermatology Discipline, Faculdade de Medicina do ABC, Santo André, SP, Brazil Received 8 December 2019; accepted 28 April 2020 Available online 24 May 2020 Abstract Vasculitis is a group of several clinical conditions in which the main histopathological KEYWORDS finding is fibrinoid necrosis in the walls of blood vessels. This article assesses the main derma- Anti-neutrophil tological aspects relevant to the clinical and laboratory diagnosis of small- and medium-vessel cytoplasmic cutaneous and systemic vasculitis syndromes. The most important aspects of treatment are also antibodies; discussed. Churg-Strauss © 2020 Sociedade Brasileira de Dermatologia. Published by Elsevier Espana,˜ S.L.U. This is an syndrome; open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). Henoch-Schönlein purple; Leukocytoclastic cutaneous vasculitis; Systemic vasculitis; Vasculitis; Vasculitis associated with lupus of the central nervous system ଝ How to cite this article: Morita TCAB, Criado PR, Criado RFJ, Trés GFS, Sotto MN. Update on vasculitis: overview and relevant dermato- logical aspects for the clinical and histopathological diagnosis --- Part II. An Bras Dermatol. 2020;95:493---507. ଝଝ Study conducted at the Department of Dermatology, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil. -

Cutaneous Vasculitides: Clinico-Pathological Correlation
Original CCutaneousutaneous vasculitides:vasculitides: Clinico-pathologicalClinico-pathological Article ccorrelationorrelation SSuruchiuruchi Gupta,Gupta, SSanjeevanjeev HHanda,anda, AmrinderAmrinder J.J. Kanwar,Kanwar, BBishanishan DDassass RadotraRadotra 1, RRanjanaanjana WW.. MMinzinz 2 Departments of Dermatology, ABSTRACT Venereology, Leprology, 1Histopathology and Background: Cutaneous vasculitis presents as a mosaic of clinical and histological 2Immunopathology, Postgraduate Institute of Þ ndings. Its pathogenic mechanisms and clinical manifestations are varied. Aims: To Medical Education and study the epidemiological spectrum of cutaneous vasculitides as seen in a dermatologic Research, Chandigarh, India clinic and to determine the clinico-pathological correlation. Methods: A cohort study was conducted on 50 consecutive patients clinically diagnosed as cutaneous vasculitis in the AAddressddress forfor ccorrespondence:orrespondence: dermatology outdoor; irrespective of age, sex and duration of the disease. Based on the Prof. Sanjeev Handa, clinical presentation, vasculitis was classiÞ ed according to modiÞ ed Gilliam’s classiÞ cation. Departments of Dermatology, All patients were subjected to a baseline workup consisting of complete hemogram, Venereology and Leprology, Postgraduate Institute of serum-creatinine levels, serum-urea, liver function tests, chest X-ray, urine (routine and Medical Education and microscopic) examination besides antistreptolysin O titer, Mantoux test, cryoglobulin levels, Research, Chandigarh, India antineutrophilic cytoplasmic antibodies and hepatitis B and C. Histopathological examination E-mail: was done in all patients while immunoß uorescence was done in 23 patients. Results: Out [email protected] of a total of 50 patients diagnosed clinically as cutaneous vasculitis, 41 were classiÞ ed as leukocytoclastic vasculitis, 2 as Heinoch−Schonlein purpura, 2 as urticarial vasculitis and one each as nodular vasculitis, polyarteritis nodosa and pityriasis lichenoid et varioliforme acuta. -

Case Report and Review of Wells' Syndrome in Childhood Amy E
Bullous “Cellulitis” With Eosinophilia: Case Report and Review of Wells’ Syndrome in Childhood Amy E. Gilliam, MD*; Anna L. Bruckner, MD‡; Rene´e M. Howard, MD*; Brian P. Lee, MD§; Susan Wu, MD§; and Ilona J. Frieden, MD* ABSTRACT. A 1-year-old girl presented with acute on- but later simplified the name to eosinophilic celluli- set of edematous erythematous plaques associated with tis.2 bullae on her extremities and accompanied by peripheral Wells’ syndrome is seen more commonly among eosinophilia. She was afebrile, and the skin lesions were adults but has been observed among children. Some pruritic but not tender. The patient was treated with hypothesize that this syndrome may represent a hy- intravenously administered antibiotics for presumed cel- persensitivity response to a circulating antigen.2 As- lulitis, without improvement. However, the lesions re- sponded rapidly to systemic steroid therapy. On the basis sociated precipitants include insect bites, medication of lesional morphologic features, peripheral eosino- reactions, recent immunization, myeloproliferative philia, and cutaneous histopathologic features, a diagno- disorders, malignancies, and infections. We describe sis of Wells’ syndrome was made. Wells’ syndrome is a case of a young child with no identifiable triggering extremely rare in childhood, with 27 pediatric cases re- factors, and we review the evidence for evaluation ported in the literature. Because it is seen so infre- and management of these pediatric cases. quently, there are no specific guidelines for evaluation and management of Wells’ syndrome among children. CASE REPORT The diagnosis should be considered for children with A previously healthy, 1-year-old girl presented with acute on- presumed cellulitis and eosinophilia who fail to respond set of edematous erythematous plaques, with associated bullae, on to antibiotics. -

Bullous Eosinophilic Annular Erythema
Volume 27 Number 5| May 2021 Dermatology Online Journal || Photo Vignette 27(5):16 Bullous eosinophilic annular erythema Yun Pei Koh1, Hong Liang Tey1-3 Affiliations: 1National Skin Centre, Singapore, 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 3Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore Corresponding Author: Koh Yun Pei, National Skin Centre, 1 Mandalay Road, Singapore 308405, Singapore, Tel: 65-2534455, Email: [email protected] areas of central clearing (Figure 1). Some plaques Abstract were studded with tense vesicles containing yellow Eosinophilic annular erythema is an idiopathic acute serous fluid, coalescing to form large bullae over his eosinophilic dermatosis. It is a rare condition, with left flank (Figure 2). Total body surface area involved approximately 30 cases reported in the English was 12%. literature. It features annular, figurate urticarial edematous plaques primarily affecting the trunk and Histological examination showed superficial and proximal limbs. During evaluation of a patient, deep perivascular infiltrate of predominantly secondary causes of eosinophilic inflammation such eosinophils and some lymphocytes (Figure 3). as allergy-related conditions (eczema, drug, urticaria, Epidermal involvement, apoptotic keratinocytes, contact dermatitis), parasitic infestations, and interface dermatitis, blistering, vasculitis, or autoimmune dermatoses will need to be excluded. granulomatous inflammation were all absent. Direct We present an unusual case of a 47-year-old patient who developed this condition. Keywords: eosinophilic dermatoses, urticaria Introduction Idiopathic primary eosinophilic dermatoses are a group of primarily eosinophil-driven skin disease characterized by moderate-to-dense eosinophilic skin infiltration with no significant infiltration of other leukocytes. Our case is consistent with one subtype of these conditions—eosinophilic annular erythema (EAE). -
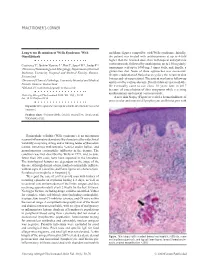
PDF Full-Text
PRACTITIONER'S CORNER Long-term Remission of Wells Syndrome With and flame figures compatible with Wells syndrome. Initially, Omalizumab the patient was treated with antihistamines at up to 4-fold higher than the licensed dose, then with topical and systemic corticosteroids, followed by azathioprine up to 150 mg daily, Coattrenec Y1, Ibrahim Yasmine L2, Harr T1, Spoerl D1*, Jandus P1* tranexamic acid up to 1000 mg 3 times daily, and, finally, a 1Division of Immunology and Allergology, Department of Internal gluten-free diet. None of these approaches was successful. Medicine, University Hospital and Medical Faculty, Geneva, Despite eradication of Helicobacter pylori, the recurrent skin Switzerland lesions and edema persisted. The patient was lost to follow-up 2Division of Clinical Pathology, University Hospital and Medical and treated by various doctors. Detailed data are not available. Faculty, Geneva, Switzerland He eventually came to our clinic 10 years later in 2017 *DS and PJ contributed equally to this work. because of exacerbation of skin symptoms while receiving antihistamines and topical corticosteroids. J Investig Allergol Clin Immunol 2020; Vol. 30(1): 58-59 doi: 10.18176/jiaci.0436 A new skin biopsy (Figure) revealed a dermal infiltrate of perivascular and interstitial lymphocytes and histiocytes with Key words: Wells syndrome. Eosinophilic cellulitis. Omalizumab. Successful treatment. Palabras clave: Síndrome Wells. Celulitis eosinofílica. Omalizumab. Tratamiento eficaz. Eosinophilic cellulitis (Wells syndrome) is an uncommon recurrent inflammatory dermatosis. It is characterized by wide clinical variability comprising itching and/or burning tender erythematous lesions, sometimes with urticaria, vesicles and/or bullae, and granulomatous eosinophilic infiltrates in the dermis. The condition was first described by Wells in 1971, and, to date, fewer than 200 cases have been reported in the literature. -

March 7, 2014
ACP AMERICAN COLLEGE OF PHYSICIANS INTERNAL MEDICINE Doctors for Adults NEW JERSEY CHAPTER AMERICAN COLLEGE OF PHYSICIANS REGIONAL SCIENTIFIC MEETING ASSOCIATES ABSTRACT COMPETITION MARCH 7, 2014 PARTICIPATING INSTITUTIONS: Atlantic Health System - Morristown Memorial Hospital - Overlook Hospital Atlanticare Regional Medical Center Barnabas Health System - Monmouth Medical Center - Newark Beth Israel Medical Center - Saint Barnabas Medical Center Capital Health System Drexel University College of Medicine, Saint Peter’s University Hospital HUMC Mountainside Hospital Meridian Health System - Jersey Shore University Medical Center Mount Sinai School of Medicine - Englewood - Jersey City Medical Center Palisades Medical Center Raritan Bay Medical Center Rowan University - Cooper Medical School - School of Osteopathic Medicine Rutgers - Robert Wood Johnson Medical School - New Jersey Medical School Seton Hall University School of Health and Sciences Medical Science - Trinitas Regional Medical Center - St. Francis Medical Center COMMITTEE MEMBERS: William E. Farrer, MD, FACP – Chairperson Neil Kothari, MD, FACP Ed Liu, MD- Co- Chairperson Michael B. Steinberg, MD, MPH, FACP Vallur Thirumavalavan, MD Shuvendu Sen, MD DISCLAIMER: It is assumed that all participants adhered to the rules as stated in the original abstract submission form. It is also assumed that the abstracts submitted were original works, represented by the true authors. The abstracts appear in no particular order. Judging was performed in an attempt to minimize bias. Judges were unaware of the authors or institutions the competitor unless they were directly involved with the associate. Although there were many excellent abstracts those selected to be presented as poster or oral presentation were chosen on the basis of content. This content was felt to be intriguing from a clinical education standpoint, thought provoking, or could stimulate debate regarding our current practice of medicine. -

Review Isolated Vasculitis of the Peripheral Nervous System
Review Isolated vasculitis of the peripheral nervous system M.P. Collins, M.I. Periquet Department of Neurology, Medical College ABSTRACT combination therapy to be more effec- of Wisconsin, Milwaukee, Wisconsin, USA. Vasculitis restricted to the peripheral tive than prednisone alone. Although Michael P. Collins, MD, Ass. Professor; nervous system (PNS), referred to as most patients have a good outcome, M. Isabel Periquet, MD, Ass. Professor. nonsystemic vasculitic neuropathy more than 30% relapse and 60% have Please address correspondence and (NSVN), has been described in many residual pain. Many nosologic, path- reprint requests to: reports since 1985 but remains a poorly ogenic, diagnostic, and therapeutic Michael P. Collins, MD, Department of understood and perhaps under-recog- questions remain unanswered. Neurology, Medical College of Wisconsin, nized condition. There are no uniform 9200 W. Wisconsin Avenue, Milwaukee, WI 53226, USA. diagnostic criteria. Classifi cation is Introduction E-mail: [email protected] complicated by the occurrence of vas- The vasculitides comprise a broad Received on March 6, 2008; accepted in culitic neuropathies in many systemic spectrum of diseases which exhibit, revised form on April 1, 2008. vasculitides affecting small-to-me- as their primary feature, infl ammation Clin Exp Rheumatol 2008; 26 (Suppl. 49): dium-sized vessels and such clinical and destruction of vessel walls, with S118-S130. variants as nonsystemic skin/nerve secondary ischemic injury to the in- © CopyrightCopyright CLINICAL AND vasculitis and diabetic/non-diabetic volved tissues (1). They are generally EXPERIMENTAL RHEUMATOLOGY 2008.2008. lumbosacral radiculoplexus neuropa- classifi ed based on sizes of involved thy. Most patients present with pain- vessels and histopathologic and clini- Key words: Vasculitis, peripheral ful, stepwise progressive, distal-pre- cal features. -

Panniculitis Martin C
Panniculitis Martin C. Mihm M.D. Director – Mihm Cutaneous Pathology Consultative Service (MCPCS) Brigham and Women’s Hospital Director – Melanoma Program Brigham and Women’s Hospital and Harvard Medical School Co-Director – Melanoma Program Dana-Farber Cancer Institute and Harvard Medical School Conflicts of Interest • Chairman Scientific Advisory Board – Caliber I.D. Inc. • Member Scientific Advisory Board – MELA Sciences Inc. • Consultant – Novartis • Consultant – Alnylam Disorders of the Subcutis • Septal • Lobular • Mixed • Inflammatory (N/G/L) • Pauci-inflammatory 1 Septal Panniculitis • Erythema nodosum • Necrobiosis lipoidica • Morphea profundus Erythema Nodosum Clinical Features • Young adults • Nodular or plaque like lesions • Anterior aspect of lower legs (common) • Arms or abdomen (occurs occasionally) • Clinical course • Initially erythematous, painful area • Evolves into nodule or plaque • Lasts 10 days to 8 weeks • Fever, malaise, arthralgias (variable s/s) Erythema Nodosum Clinical Features Causation • Systemic diseases: CTD, Behcet’s, Sweet’s, sarcoidosis,etc. • Drugs: Numerous drugs have been associated: penicillin, sulfa, Cipro, isotretinoin, etc. • 30%: idiopathic or of unknown cause.. 2 3 Erythema nodosum : Well Developed Lesion • Septal fibrosis • Septal chronic inflammation • Lymphocytes • Frank Vasculitis may not be present • Granulomatous changes • Small granulomatous aggregates of histiocytes • Miescher’s radial granuloma • Multinucleated giant cells 4 5 6 Erythema nodosum : Morphologic Clues to underlying etiology