Lyme Bay - a Case Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Ecosystem Model of the North Sea to Support an Ecosystem Approach to Fisheries Management: Description and Parameterisation
Science Series Technical Report no.142 An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation S. Mackinson and G. Daskalov Science Series Technical Report no.142 An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation S. Mackinson and G. Daskalov This report should be cited as: Mackinson, S. and Daskalov, G., 2007. An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation. Sci. Ser. Tech Rep., Cefas Lowestoft, 142: 196pp. This report represents the views and findings of the authors and not necessarily those of the funders. © Crown copyright, 2008 This publication (excluding the logos) may be re-used free of charge in any format or medium for research for non-commercial purposes, private study or for internal circulation within an organisation. This is subject to it being re-used accurately and not used in a misleading context. The material must be acknowledged as Crown copyright and the title of the publication specified. This publication is also available at www.Cefas.co.uk For any other use of this material please apply for a Click-Use Licence for core material at www.hmso.gov.uk/copyright/licences/ core/core_licence.htm, or by writing to: HMSO’s Licensing Division St Clements House 2–16 Colegate Norwich NR3 1BQ Fax: 01603 723000 E-mail: [email protected] List of contributors and reviewers Name Affiliation -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -
Sparus Aurata Global Invasive Species Database (GISD)
FULL ACCOUNT FOR: Sparus aurata Sparus aurata System: Marine_freshwater_brackish Kingdom Phylum Class Order Family Animalia Chordata Actinopterygii Perciformes Sparidae Common name snapper (English, New Zealand), gilthead (English), gilt head (English), gilt head bream (English), gilthead bream (English), gilt- head seabream (English), goudbrasem (Dutch), kultaotsa-ahven (Finnish), n'tad (Arabic), orada (Catalan), orada (Croatian), silver seabream (English), daurade royale (French, Mauritania), dorade (French), Dorade (German), dorade royale (French), Dorade Royal (German), Gemeine Goldbrasse (German), Goldbrasse (German), goldbrassen (German), Goldkopf (German), daurade (French), tsipoura (Greek), dorada (Spanish), dourada (Portuguese), cipura (Turkish), goud brasem (Dutch), komarca (Croatian), lovrata (Croatian), ovrata (Croatian), podlanica (Croatian), dinigla (Croatian), væbnerfisk (Danish), guldbrasen (Danish) Synonym Aurata aurata , (Linnaeus, 1758) Chrysophrys aurata , (Linnaeus, 1758) Chrysophrys aurathus , (Linnaeus, 1758) Chrysophrys auratus , (Linnaeus, 1758) Chrysophrys crassirostris , Valenciennes, 1830 Pagrus auratus , (Linnaeus, 1758) Pagrus auratus , (non Forster, 1801) Sparus auratus , Linnaeus, 1758 Similar species Summary Gilthead bream (Sparus aurata) is a fish of Mediterranean and Atlantic Ocean origin. It is one of the most important fish in the aquaculture industry in the Mediterranean. However the rapid development of marine cage culture of this fish has raised concerns about the impact of escaped fish on the genetic diversity of natural populations. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Sparus aurata. Pag. 1 Available from: http://www.iucngisd.org/gisd/species.php?sc=1703 [Accessed 02 October 2021] FULL ACCOUNT FOR: Sparus aurata Species Description The gilthead bream is a Mediterranean fish reaching a maximum of 70 cm length and 6 kg in weight (Balart et al., 2009). -

For Review Only 19 20 21 504 Ampuero D, T
Page 1 of 39 Zoological Journal of the Linnean Society 1 2 3 1 DNA identification and larval morphology provide new evidence on the systematic 4 5 2 position of Ergasticus clouei A. Milne-Edwards, 1882 (Decapoda, Brachyura, 6 7 3 Majoidea) 8 9 10 4 11 1 2 1 3 12 5 Marco-Herrero, Elena , Torres, Asvin P. , Cuesta, José A. , Guerao, Guillermo , Palero, 13 14 6 Ferran 4, & Abelló, Pere 5 15 16 7 17 18 8 1Instituto de CienciasFor Marinas Review de Andalucía (ICMAN-C OnlySIC), Avda. República 19 20 21 9 Saharaui, 2, 11519 Puerto Real, Cádiz, Spain. 22 2 23 10 Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent 24 25 11 s/n, 07015 Palma, Spain. 26 27 12 3IRTA, Unitat de Cultius Aqüàtics. Ctra. Poble Nou, Km 5.5, 43540 Sant Carles de la 28 29 30 13 Ràpita, Tarragona, Spain. 31 4 32 14 Unitat Mixta Genòmica i Salut CSISP-UV, Institut Cavanilles Universitat de Valencia, 33 34 15 C/ Catedrático José Beltrán 2, 46980 Paterna, Spain. 35 36 16 5Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta 37-49, 08003 37 38 17 Barcelona, Catalonia. Spain. 39 40 41 18 42 43 19 44 45 20 46 47 21 RUN TITLE: Larval evidence and the systematic position of Ergasticus clouei 48 49 50 22 51 52 53 54 55 56 57 58 59 60 Zoological Journal of the Linnean Society Page 2 of 39 1 2 3 23 ABSTRACT: The morphology of the complete larval stage series of the crab Ergasticus 4 5 24 clouei is described and illustrated based on larvae (zoea I, zoea II and megalopa) 6 7 25 captured from plankton samples taken in Mediterranean waters. -

Marine Fishes from Galicia (NW Spain): an Updated Checklist
1 2 Marine fishes from Galicia (NW Spain): an updated checklist 3 4 5 RAFAEL BAÑON1, DAVID VILLEGAS-RÍOS2, ALBERTO SERRANO3, 6 GONZALO MUCIENTES2,4 & JUAN CARLOS ARRONTE3 7 8 9 10 1 Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca 11 e Asuntos Marítimos, Rúa do Valiño 63-65, 15703 Santiago de Compostela, Spain. E- 12 mail: [email protected] 13 2 CSIC. Instituto de Investigaciones Marinas. Eduardo Cabello 6, 36208 Vigo 14 (Pontevedra), Spain. E-mail: [email protected] (D. V-R); [email protected] 15 (G.M.). 16 3 Instituto Español de Oceanografía, C.O. de Santander, Santander, Spain. E-mail: 17 [email protected] (A.S); [email protected] (J.-C. A). 18 4Centro Tecnológico del Mar, CETMAR. Eduardo Cabello s.n., 36208. Vigo 19 (Pontevedra), Spain. 20 21 Abstract 22 23 An annotated checklist of the marine fishes from Galician waters is presented. The list 24 is based on historical literature records and new revisions. The ichthyofauna list is 25 composed by 397 species very diversified in 2 superclass, 3 class, 35 orders, 139 1 1 families and 288 genus. The order Perciformes is the most diverse one with 37 families, 2 91 genus and 135 species. Gobiidae (19 species) and Sparidae (19 species) are the 3 richest families. Biogeographically, the Lusitanian group includes 203 species (51.1%), 4 followed by 149 species of the Atlantic (37.5%), then 28 of the Boreal (7.1%), and 17 5 of the African (4.3%) groups. We have recognized 41 new records, and 3 other records 6 have been identified as doubtful. -
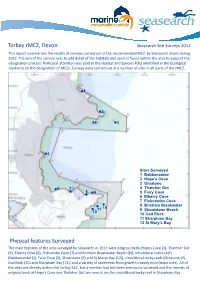
Torbay Rmcz, Devon Physical Features Surveyed
Torbay rMCZ, Devon Seasearch Site Surveys 2012 This report summarises the results of surveys carried out in the recommended MCZ by Seasearch divers during 2012. The aim of the surveys was to add detail of the habitats and species found within the area to support the designation process. Particular attention was paid to the Habitat and Species FOCI identified in the Ecological Guidance on the designation of MCZs. Surveys were carried out at a number of sites in all parts of the rMCZ. 1 2 4 3 5 Sites Surveyed 1 Babbacombe 2 Hope’s Cove CW 3 Orestone 6 7 4 Thatcher Gut 8 9 5 Fairy Cove 6 Elberry Cove 7 Fishcombe Cove 8 Brixham Breakwater 10 11 9 Shoalstone Beach 12 10 Cod Rock 11 Sharpham Bay 12 St Mary’s Bay Physical features Surveyed The main features of the area surveyed by Seasearch in 2012 were eelgrass beds (Hopes Cove (2), Thatcher Gut (4), Elberry Cove (6), Fishcombe Cove (7) and Brixham Breakwater Beach (8)), infralittoral rocky reefs (Babbacombe (1), Fairy Cove (5), Shoalstone (9) and St Marys Bay (12)), circalittoral rocky reefs (Orestone (3), Cod Rock (10) and Sharpham Bay (11)) and a variety of sediments from gravel to sandy mud (most sites). All of the sites are already within the Torbay SAC, but a number had not been previously surveyed and the records of eelgrass beds at Hope’s Cove and Thatcher Gut are new, is arCWe the circalittoral rocky reef in Sharpham Bay. CW Features of the Marine Life and Habitats Eeelgrass Beds (Sites 2,4,6,7,8) Eelgrass, Zostera marina, is one of the very few flowering plants underwater and, unlike seaweeds, has a system of roots which can help to bind soft sediments and create a complex habitat in what would otherwise be a relatively un-diverse environment. -

Decapoda, Brachyura
APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA de Decápodos Braquiuros de la Península Ibérica bérica I enínsula P raquiuros de la raquiuros B ecápodos D de APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA LA DE IDENTIFICACIÓN EN LA Y MOLECULARES MORFOLÓGICAS TÉCNICAS DE APLICACIÓN Herrero - MEGALOPA “big eyes” Leach 1793 Elena Marco Elena Marco-Herrero Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 Tesis Doctoral, Valencia 2015 Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA DE DECÁPODOS BRAQUIUROS DE LA PENÍNSULA IBÉRICA TESIS DOCTORAL Elena Marco-Herrero Valencia, septiembre 2015 Directores José Antonio Cuesta Mariscal / Ferran Palero Pastor Tutor Álvaro Peña Cantero Als naninets AGRADECIMIENTOS-AGRAÏMENTS Colaboración y ayuda prestada por diferentes instituciones: - Ministerio de Ciencia e Innovación (actual Ministerio de Economía y Competitividad) por la concesión de una Beca de Formación de Personal Investigador FPI (BES-2010- 033297) en el marco del proyecto: Aplicación de técnicas morfológicas y moleculares en la identificación de estados larvarios planctónicos de decápodos braquiuros ibéricos (CGL2009-11225) - Departamento de Ecología y Gestión Costera del Instituto de Ciencias Marinas de Andalucía (ICMAN-CSIC) - Club Náutico del Puerto de Santa María - Centro Andaluz de Ciencias y Tecnologías Marinas (CACYTMAR) - Instituto Español de Oceanografía (IEO), Centros de Mallorca y Cádiz - Institut de Ciències del Mar (ICM-CSIC) de Barcelona - Institut de Recerca i Tecnología Agroalimentàries (IRTA) de Tarragona - Centre d’Estudis Avançats de Blanes (CEAB) de Girona - Universidad de Málaga - Natural History Museum of London - Stazione Zoologica Anton Dohrn di Napoli (SZN) - Universitat de Barcelona AGRAÏSC – AGRADEZCO En primer lugar quisiera agradecer a mis directores, el Dr. -

A History of the British Stalk-Eyed Crustacea
Go ygle Acerca de este libro Esta es una copia digital de un libro que, durante generaciones, se ha conservado en las estanterias de una biblioteca, hasta que Google ha decidido escanearlo como parte de un proyecto que pretende que sea posible descubrir en linea libros de todo el mundo. Ha sobrevivido tantos anos como para que los derechos de autor hayan expirado y el libro pase a ser de dominio publico. El que un libro sea de dominio publico significa que nunca ha estado protegido por derechos de autor, o bien que el periodo legal de estos derechos ya ha expirado. Es posible que una misma obra sea de dominio publico en unos paises y, sin embargo, no lo sea en otros. Los libros de dominio publico son nuestras puertas hacia el pasado, suponen un patrimonio historico, cultural y de conocimientos que, a menudo, resulta dificil de descubrir. Todas las anotaciones, marcas y otras senales en los margenes que esten presentes en el volumen original apareceran tambien en este archivo como testimonio del largo viaje que el libro ha recorrido desde el editor hasta la biblioteca y, finalmente, hasta usted. Normas de uso Google se enorgullece de poder colaborar con distintas bibliotecas para digitalizar los materiales de dominio publico a fin de hacerlos accesibles a todo el mundo. Los libros de dominio publico son patrimonio de todos, nosotros somos sus humildes guardianes. No obstante, se trata de un trabajo caro. Por este motivo, y para poder ofrecer este recurso, hemos tornado medidas para evitar que se produzca un abuso por parte de terceros con fines comerciales, y hemos incluido restricciones tecnicas sobre las solicitudes automatizadas. -

Larval Growth
LARVAL GROWTH Edited by ADRIAN M.WENNER University of California, Santa Barbara OFFPRINT A.A.BALKEMA/ROTTERDAM/BOSTON DARRYL L.FELDER* / JOEL W.MARTIN** / JOSEPH W.GOY* * Department of Biology, University of Louisiana, Lafayette, USA ** Department of Biological Science, Florida State University, Tallahassee, USA PATTERNS IN EARLY POSTLARVAL DEVELOPMENT OF DECAPODS ABSTRACT Early postlarval stages may differ from larval and adult phases of the life cycle in such characteristics as body size, morphology, molting frequency, growth rate, nutrient require ments, behavior, and habitat. Primarily by way of recent studies, information on these quaUties in early postlarvae has begun to accrue, information which has not been previously summarized. The change in form (metamorphosis) that occurs between larval and postlarval life is pronounced in some decapod groups but subtle in others. However, in almost all the Deca- poda, some ontogenetic changes in locomotion, feeding, and habitat coincide with meta morphosis and early postlarval growth. The postmetamorphic (first postlarval) stage, here in termed the decapodid, is often a particularly modified transitional stage; terms such as glaucothoe, puerulus, and megalopa have been applied to it. The postlarval stages that fol low the decapodid successively approach more closely the adult form. Morphogenesis of skeletal and other superficial features is particularly apparent at each molt, but histogenesis and organogenesis in early postlarvae is appreciable within intermolt periods. Except for the development of primary and secondary sexual organs, postmetamorphic change in internal anatomy is most pronounced in the first several postlarval instars, with the degree of anatomical reorganization and development decreasing in each of the later juvenile molts. -

ECOLOGIA POPULACIONAL DE Libinia Ferreirae (BRACHYURA: MAJOIDEA) NO LITORAL SUDESTE DO BRASIL
UNIVERSIDADE ESTADUAL PAULISTA INSTITUTO DE BIOCIÊNCIAS GESLAINE RAFAELA LEMOS GONÇALVES ECOLOGIA POPULACIONAL DE Libinia ferreirae (BRACHYURA: MAJOIDEA) NO LITORAL SUDESTE DO BRASIL DISSERTAÇÃO DE MESTRADO BOTUCATU 2016 UNIVERSIDADE ESTADUAL PAULISTA INSTITUTO DE BIOCIÊNCIAS DISSERTAÇÃO DE MESTRADO Ecologia populacional de Libinia ferreirae (Brachyura: Majoidea) no litoral sudeste do Brasil Geslaine Rafaela Lemos Gonçalves Orientador: Prof. Dr. Antonio Leão Castilho Coorientadora: Profª. Drª. Maria Lucia Negreiros Fransozo Dissertação apresentada ao Instituto de Biociências da Universidade Estadual Paulista “Júlio de Mesquita Filho” – UNESP – Câmpus Botucatu, como parte dos requisitos para obtenção do Título de Mestre em Ciências, curso de Pós-graduação em Ciências Biológicas, Área de Concentração: Zoologia. Botucatu – SP 2016 FICHA CATALOGRÁFICA ELABORADA PELA SEÇÃO TÉC. AQUIS. TRATAMENTO DA INFORM. DIVISÃO TÉCNICA DE BIBLIOTECA E DOCUMENTAÇÃO - CÂMPUS DE BOTUCATU - UNESP BIBLIOTECÁRIA RESPONSÁVEL: ROSEMEIRE APARECIDA VICENTE-CRB 8/5651 Gonçalves, Geslaine Rafaela Lemos. Ecologia populacional de Libinia ferreirae (Brachyura: Majoidea) no litoral sudeste do Brasil / Geslaine Rafaela Lemos Gonçalves. - Botucatu, 2016 Dissertação (mestrado) - Universidade Estadual Paulista "Júlio de Mesquita Filho", Instituto de Biociências de Botucatu Orientador: Antonio Leão Castilho Coorientador: Maria Lucia Negreiros Fransozo Capes: 20402007 1. Caranguejo. 2. Dinâmica populacional. 3. Hábitos alimentares. 4. Ecologia de populações. 5. Epizoísmo. Palavras-chave: Ciclo de vida; Crescimento; Dinâmica populacional; Epizoísmo; Hábitos alimentares. NEBECC Núcleo de Estudos em Biologia, Ecologia e Cultivo de Crustáceos II “Sou biólogo e viajo pela savana de meu país. Nessa região encontro gente que não sabe ler livros. Mas que sabe ler o mundo. Nesse universo de outros saberes, sou eu o analfabeto” Mia Couto “O saber a gente aprende com os mestres e com os livros. -

Post-Larval Developm Ent and Sexual Dim Orphism of the Spider Crab M
SciENTiA M arina 73(4) December 2009, 797-808, Barcelona (Spain) ISSN: 0214-8358 doi: 10.3989/scimar.2009.73n4797 Post-larval development and sexual dimorphism of the spider crab Maja brachydactyla (Brachyura: Majidae) GUILLERMO GUERAO and GUIOMAR ROTLLANT IRTA, Unitat de Cultius Experimentáis. Ctra. Pohle Nou, km 5.5, 43540 Sant Caries de la Rápita, Tarragona, Spain. E-mail: [email protected] SUMMARY: The post-larval development of the majid crab Maja brachydactyla Balss, 1922 was studied using laboratory- reared larvae obtained from adult individuals collected in the NE Atlantic. The morphology of the first juvenile stage is described in detail, while the most relevant morphological changes and sexual differentiation are highlighted for subsequent juvenile stages, until juvenile 8. The characteristic carapace spines of the adult phase are present in the first juvenile stage, though with great differences in the degree of development and relative size. The carapace shows a high length/weight ratio, which becomes similar to that of adults at stage 7-8. Males and females can be distinguished from juvenile stage 4. based on sexual dimorphism in the pleopods and the presence of gonopores. In addition, the allometric growth of the pleon is sex-dependent from juvenile stage 4. with females showing a positive allometry (b=1.23) and males an isometric allometry (b=1.02). Keywords'. Majidae. Maja brachydactyla, morphology, juvenile, post-larval development, growth, sexual dimorphism. RESUMEN: D esarrollo postlarvario y dimorfismo sexual de M a j a brachydactyia (Brachyura: M ajidae). - El desarrollo postlarvario del májido Maja brachydactyla ha sido estudiado en el laboratorio después del cultivo larvario realizado a partir de individuos adultos capturados en el NE del Atlántico. -

Edinburgh Research Explorer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Edinburgh Research Explorer Edinburgh Research Explorer Some observations on the Mesolithic crustacean assemblage from Ulva Cave, Inner Hebrides, Scotland. Citation for published version: Pickard, C & Bonsall, C 2009, 'Some observations on the Mesolithic crustacean assemblage from Ulva Cave, Inner Hebrides, Scotland.'. in JM Burdukiewicz, K Cyrek, P Dyczek & K Szymczak (eds), Understanding the Past: Papers Offered to Stefan K. Kozowski. University of Warsaw Center for Research on the Antiquity of Southeastern Europe, Warsaw, pp. 305-313. Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher final version (usually the publisher pdf) Published In: Understanding the Past Publisher Rights Statement: © Pickard, C., & Bonsall, C. (2009). Some observations on the Mesolithic crustacean assemblage from Ulva Cave, Inner Hebrides, Scotland.In J. M. Burdukiewicz, K. Cyrek, P. Dyczek, & K. Szymczak (Eds.), Understanding the Past. (pp. 305-313). Warsaw: University of Warsaw Center for Research on the Antiquity of Southeastern Europe. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim.