PREDICTORS of POST-STROKE FATIGUE by Benjamin Yichen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SOS – Save Our Shoulders: a Guide for Polio Survivors
1 • Save Our Shoulders: A Guide for Polio Survivors A Guide for Polio Survivors S.O.S. Save Our Shoulders: A Guide for Polio Survivors by Jennifer Kuehl, MPT Roberta Costello, MSN, RN Janet Wechsler, PT Funding for the production of this manual was made possible by: The National Institute for Disability and Rehabilitation Research Grant #H133A000101 and The U.S. Department of the Army Grant #DAMD17-00-1-0533 Investigators: Mary Klein, PhD Mary Ann Keenan, MD Alberto Esquenazi, MD Acknowledgements We gratefully acknowledge the contributions and input provided from all of those who participated in our research. The time and effort of our participants was instrumental in the creation of this manual. Jennifer Kuehl, MPT Moss Rehabilitation Research Institute, Philadelphia Roberta Costello, MSN, RN Moss Rehabilitation Research Institute, Philadelphia Janet Wechsler, PT Moss Rehabilitation Research Institute, Philadelphia Mary Klein, PhD Moss Rehabilitation Research Institute, Philadelphia Mary Ann Keenan, MD University of Pennsylvania, Philadelphia Alberto Esquenazi, MD MossRehab Hospital, Philadelphia Cover and manual design by Ron Kalstein, MEd Albert Einstein Medical Center, Philadelphia Table of Contents 1. Introduction . .5 2. General Information About the Shoulder . .6 3. Facts About Shoulder Problems . .8 4. Treatment Options . .13 5. About Exercise . .16 6. Stretching Exercises . .19 7. Cane Stretches . .22 8. Strengthening Exercises . .25 9. Tips to Avoid Shoulder Problems . .29 10. Conclusion . .31 11. Resources . .31 The information contained within this manual is for reference only and is not a substitute for professional medical advice. Before beginning any exercise program consult your physician. Save Our Shoulders: A Guide for Polio Survivors • 4 Introduction Many polio survivors report new symptoms as they age. -

Fatigue After Stroke
SPECIAL REPORT Fatigue after stroke PJ Tyrrell Fatigue is a common symptom after stroke. It is not invariably related to stroke severity & DG Smithard† and can occur in the absence of depression. It is one of the most troublesome symptoms for †Author for correspondence many patients and yet nothing is known of its causation. There are no specific treatments. William Harvey Hospital, Richard Steven’s Ward, This article assesses the available literature in the context of what is known about fatigue Ashford, Kent, in other disorders. Post-stroke fatigue may be a manifestation of sickness behavior, TN24 0LZ, UK mediated through the central effects of the cytokine interleukin-1, perhaps via effects on Tel.: +44 123 361 6214 Fax: +44 123 361 6662 glutamate neurotransmission. Possible therapeutic strategies are discussed which might be [email protected] a logical basis from which to plan randomized control trials. Following stroke, approximately a third of common and disabling symptom of Parkinson’s patients die, a third recover and a third remain disease [7,8] and of systemic lupus [9]. More than significantly disabled. Even those who recover 90% of patients with poliomyelitis develop a physically may be left with significant emotional delayed syndrome of post-myelitis fatigue [10]. and psychologic dysfunction – including anxi- Fatigue is the most prevalent symptom of ety, readjustment reactions and depression. One patients with cancer who receive radiation, cyto- common but often overlooked symptom is toxic or other therapies [11], and it may persist fatigue. This may occur soon or late after stroke, for years after the cessation of treatment [12]. -
Stroke: Learn the Warning Signs and Protect Yourself Strokes Are the Leading Cause of Major Disability in the U.S
stroke: learn the warning signs and protect yourself Strokes are the leading cause of major disability in the U.S. and the second leading cause of death worldwide. With all the emphasis placed on heart disease reaches us, they’ve already been to the hospital. The and cancer as the two leading causes of death in only thing left by then is active physical therapy.” America, people often forget what ranks as third. The answer is stroke, and it can have debilitating Dr. Ray says that is why he preaches religiously effects even if you survive. to patients about lowering blood pressure and cholesterol, healthy diet and regular exercise, and On average, someone has a stroke in the U.S. every keeping any pre-existing conditions in check through 45 seconds, which equals 700,000 strokes a year. regular checkups. He goes on to say that having a Of these, about 500,000 are first-time strokes. primary care physician is vital. Strokes are so pervasive that they are the leading cause of major disability in the “All adults should speak to their doctors U.S. and the second leading cause of death about diet and controlling their blood worldwide. pressure,” Dr. Ray says. “You want to actively prevent this before it happens.” A stroke occurs when blood flow to parts of the brain is restricted or impaired, There are two basic types of stroke. The first killing off brain cells. Those cells can never is ischemic stroke caused by blood clots or regenerate, which means the damage is other particles in the blood vessels leading permanent. -

Epilepsy After Stroke
Stroke Helpline: 0303 3033 100 Website: stroke.org.uk Epilepsy after stroke In the first few weeks after a stroke some people have a seizure, and a small number go on to develop epilepsy – a tendency to have repeated seizures. These can usually be completely controlled with treatment. This factsheet explains what epilepsy is, the different types of seizures, and how epilepsy is diagnosed and treated. It also includes advice about coping with a seizure, and a glossary. Epilepsy is a tendency to have repeated What causes seizures? seizures – sometimes called ‘fits’ or ‘attacks’. It affects just under one per cent Cells in the brain communicate with one of people in the UK. Stroke is one of many another and with our muscles by passing conditions that can lead to epilepsy. electrical signals along nerve fibres. If you have epilepsy this electrical activity can Around five per cent of people who have a become disordered. A sudden abnormal stroke will have a seizure within the following burst of electrical activity in the brain can few weeks. These are known as acute or lead to a seizure. onset seizures and normally happen within 24 hours of the stroke. You are more likely There are over 40 different types of seizures to have one if you have had a severe stroke, ranging from tingling sensations or ‘going a stroke caused by bleeding in the brain (a blank’ for a few seconds, to shaking and haemorrhagic stroke), or a stroke involving losing consciousness. the part of the brain called the cerebral cortex. If you have an onset seizure, it This can mean that epilepsy is sometimes does not necessarily mean you have or will confused with other conditions, including develop epilepsy. -

Acute Ischemic Stroke in Young Adults with Tuberculous Meningitis Liming Zhang1, Xiaoyu Zhang2, Huaqiang Li1,3, Gang Chen1* and Meijia Zhu2*
Zhang et al. BMC Infectious Diseases (2019) 19:362 https://doi.org/10.1186/s12879-019-4004-5 RESEARCHARTICLE Open Access Acute ischemic stroke in young adults with tuberculous meningitis Liming Zhang1, Xiaoyu Zhang2, Huaqiang Li1,3, Gang Chen1* and Meijia Zhu2* Abstract Background: Ischemic stroke is a common complication in patients with tuberculous meningitis (TBM), which is associated with poor clinical outcome. However, risk factors of stroke in TBM patients were not fully understood, especially in those young adults. Therefore, the aim of our study was to identify risk factors for acute ischemic stroke in young adults with TBM. Methods: TBM patients (18 to 50 years) without cerebral vascular risk factors were prospective recruited between Feb 2014 and Dec 2017. Patients were defined as stroke group and non-stroke group by brain magnetic resonance imaging (MRI). Demographic characteristics, clinical presentations, cerebrospinal fluid (CSF) examination, basal meningeal enhancement, hydrocephalus, tuberculoma and clinical outcome were compared between two groups. Binary logistic regression was performed to determine risk factors for acute ischemic stroke in young TBM patients. Results: Fifty-two patients with TBM were included and 12 (23.1%) patients were in stroke group. Patients in stroke group were older. Clinical presentations were comparable between two groups except headache was more common in TBM patients with stroke. In CSF examination, TBM patients with stroke had higher CSF white blood cell. By MRI, patients in stroke group were more likely to have basal meningeal enhancement but less likely to present tuberculoma. Compared to non-stroke group, patients in stroke group had worse short-term clinical outcome. -

Bacterial Meningitis and Neurological Complications in Adults
OCUSED EVIEW Parunyou J. et al. Bacterial meningitis F R Bacterial meningitis and neurological complications in adults Parunyou Julayanont MD, Doungporn Ruthirago MD, John C. DeToledo MD ABSTRACT Bacterial meningitis is a leading cause of death from infectious disease worldwide. The neurological complications secondary to bacterial meningitis contribute to the high mortality rate and to disability among the survivors. Cerebrovascular complications, including infarc- tion and hemorrhage, are common. Inflammation and increased pressure in the subarach- noid space result in cranial neuropathy. Seizures occur in either the acute or delayed phase after the infection and require early detection and treatment. Spreading of infection to other intracranial structures, including the subdural space, brain parenchyma, and ventricles, in- creases morbidity and mortality in survivors. Infection can also spread to the spinal canal causing spinal cord abscess, epidural abscess, polyradiculitis, and spinal cord infarction secondary to vasculitis of the spinal artery. Hypothalamic-pituitary dysfunction is also an un- common complication after bacterial meningitis. Damage to cerebral structures contributes to cognitive and neuropsychiatric problems. Being aware of these complications leads to early detection and treatment and improves mortality and outcomes in patients with bacte- rial meningitis. Key words: meningitis; meningitis, bacterial; central nervous system bacterial infection; nervous system diseases INTRODUCTION prove recovery and outcomes. Bacterial meningitis is a leading cause of death In this article, we present a case of bacterial men- from infectious disease worldwide. Despite the avail- ingitis complicated by an unusual number of neuro- ability of increasingly effective antibiotics and inten- logical complications that occurred in spite of a timely sive neurological care, the overall mortality remains diagnosis, adequate treatment, and intensive neuro- high, with 17-34% of the survivors having unfavorable logical monitoring. -
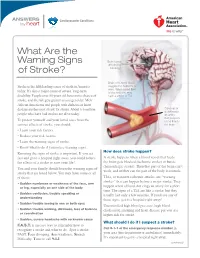
What Are the Warning Signs of Stroke? (PDF)
ANSWERS Cardiovascular Conditions by heart What Are the Warning Signs Brain tissue affected by of Stroke? blockage Brain cells need blood, Stroke is the fifth leading cause of death in America oxygen and nutrients to work. When blood flow today. It’s also a major cause of severe, long-term is blocked, you may disability. People over 55 years old have more chance of have a stroke or TIA. stroke, and the risk gets greater as you get older. Men, African Americans and people with diabetes or heart disease are the most at risk for stroke. About 6.6 million Close-up of a clot inside people who have had strokes are alive today. an artery that provides To protect yourself and your loved ones from the blood flow to serious effects of stroke, you should: the brain • Learn your risk factors. • Reduce your risk factors. • Learn the warning signs of stroke. • Know what to do if you notice warning signs. How does stroke happen? Knowing the signs of stroke is important. If you act fast and go to a hospital right away, you could reduce A stroke happens when a blood vessel that feeds the effects of a stroke or save your life! the brain gets blocked (ischemic stroke) or bursts (hemorrhagic stroke). Then that part of the brain can’t You and your family should learn the warning signs of work, and neither can the part of the body it controls. stroke that are listed below. You may have some or all of them: TIAs, or transient ischemic attacks, are “warning strokes” that can happen before a major stroke. -

Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives
International Journal of Molecular Sciences Review Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives Diji Kuriakose and Zhicheng Xiao * Development and Stem Cells Program, Monash Biomedicine Discovery Institute and Department of Anatomy and Developmental Biology, Monash University, Melbourne, VIC 3800, Australia; [email protected] * Correspondence: [email protected] Received: 29 September 2020; Accepted: 13 October 2020; Published: 15 October 2020 Abstract: Stroke is the second leading cause of death and a major contributor to disability worldwide. The prevalence of stroke is highest in developing countries, with ischemic stroke being the most common type. Considerable progress has been made in our understanding of the pathophysiology of stroke and the underlying mechanisms leading to ischemic insult. Stroke therapy primarily focuses on restoring blood flow to the brain and treating stroke-induced neurological damage. Lack of success in recent clinical trials has led to significant refinement of animal models, focus-driven study design and use of new technologies in stroke research. Simultaneously, despite progress in stroke management, post-stroke care exerts a substantial impact on families, the healthcare system and the economy. Improvements in pre-clinical and clinical care are likely to underpin successful stroke treatment, recovery, rehabilitation and prevention. In this review, we focus on the pathophysiology of stroke, major advances in the identification of therapeutic targets and recent trends in stroke research. Keywords: stroke; pathophysiology; treatment; neurological deficit; recovery; rehabilitation 1. Introduction Stroke is a neurological disorder characterized by blockage of blood vessels. Clots form in the brain and interrupt blood flow, clogging arteries and causing blood vessels to break, leading to bleeding. -

Life-After-Stroke-Guide 7819.Pdf
A GUIDE FOR PATIENTS AND CAREGIVERS LIFE AFTER STROKE Our Path Forward Encompass Health is a national sponsor of Together to End Stroke. TABLE OF CONTENTS 04 What is a stroke? 24 Rehabilitation setting options 06 About my stroke 25 Tips for choosing a rehabilitation facility 07 Diagnosis and early treatment 26 What to expect in rehabilitation 10 Common physical changes after a stroke 29 My rehabilitation goals 13 Common communication and cognitive changes 30 Preventing another after stroke stroke 18 Common emotional and 33 Signs and symptoms of personality changes after stroke stroke 34 Resources 22 Why rehabilitation is important INTRODUCTION THERE IS LIFE – AND HOPE – AFTER STROKE. WITH TIME, NEW ROUTINES WILL BECOME SECOND NATURE. REHABILITATION CAN BUILD YOUR STRENGTH, CAPABILITY AND CONFIDENCE. IT CAN HELP YOU CONTINUE YOUR DAILY ACTIVITIES DESPITE THE EFFECTS OF YOUR STROKE. If you are the caregiver, family member or friend of a stroke survivor, your role is vital. You should know the prevention plan and help your loved one to comply with the plan. With a committed health care team and a rehabilitation plan specific to their needs, most stroke survivors can prevent another stroke and thrive. We hope this guide will help you and your loved ones understand the effects of stroke and how to maximize your rehabilitation and recovery. 03 WHAT IS A STROKE? Stroke is an event that affects the arteries of the brain. A stroke occurs when a blood vessel bringing blood to the brain gets blocked or ruptures (bursts). This means that the area of the brain the blocked or ruptured blood vessel supplies can’t get the oxygen and nutrients it needs. -

Vaccine Injury Table
Vaccine Injury Table Applies Only to Petitions for Compensation Filed under the National Vaccine Injury Compensation Program on or after March 21, 2017 (a) In accordance with section 312(b) of the National Childhood Vaccine Injury Act of 1986, title III of Public Law 99-660, 100 Stat. 3779 (42 U.S.C. 300aa-1 note) and section 2114(c) of the Public Health Service Act, as amended (PHS Act) (42 U.S.C. 300aa-14(c)), the following is a table of vaccines, the injuries, disabilities, illnesses, conditions, and deaths resulting from the administration of such vaccines, and the time period in which the first symptom or manifestation of onset or of the significant aggravation of such injuries, disabilities, illnesses, conditions, and deaths is to occur after vaccine administration for purposes of receiving compensation under the Program. Paragraph (b) of this section sets forth additional provisions that are not separately listed in this Table but that constitute part of it. Paragraph (c) of this section sets forth the qualifications and aids to interpretation for the terms used in the Table. Conditions and injuries that do not meet the terms of the qualifications and aids to interpretation are not within the Table. Paragraph (d) of this section sets forth a glossary of terms used in paragraph (c). Time period for first symptom or manifestation of onset or of Illness, disability, injury significant aggravation after Vaccine or condition covered vaccine administration I. Vaccines containing tetanus toxoid (e.g., A. Anaphylaxis ≤4 hours. DTaP, DTP, DT, Td, or TT) B. Brachial Neuritis 2-28 days (not less than 2 days and not more than 28 days). -

Seizures Post Stroke
Seizures As a Consequence of Stroke Denise S. Taylor, DO Athens Neurological Associates Disclosure • I have the following financial relationship to report: – UCB Speakers Bureau Learning Objectives 1. Identify patients at risk for post stroke seizure. 2. Identify seizure type and describe proper work up. 3. Describe treatment options for acute seizure post stroke. Impact of Stroke • Stroke is the 5th leading cause of death in men and the 3rd leading cause of death for women. • It remains among the top 10 causes of death in children. • Stroke is on the rise among younger adults – 15% of ischemic strokes occur in young adults and adolescents. • Stroke is the leading cause of adult disability. National Stroke Association; 2017 Stroke Types • Ischemic Stroke – Thrombotic - large vessel is the most common – Embolic - 15% of embolic strokes occur in people with atrial fibrillation • Hemorrhagic strokes are less common, only 15% of all strokes are hemorrhagic, but they are responsible for about 40% of all stroke deaths. National Stroke Association; 2017 Seizures Post-Stroke • In the first few weeks following a stroke some stroke survivors will experience a seizure. • Seizures occur due to sudden disorganized electrical activity in the brain. • Stroke is the most common cause of seizures in older people. • Around 5% of people will have a seizure within a few weeks of having a stroke. • It is difficult to predict which stroke survivors will have a seizure. • Acute seizures normally happen within 24 hours of the stroke. • Increased risk is associated with hemorrhagic stroke or large cortical stroke. • Many clinical studies make a distinction between early and late seizures based on differences in their presumed pathophysiology. -

Dissecting the Association Between Migraine and Stroke
Curr Neurol Neurosci Rep (2015) 15:5 DOI 10.1007/s11910-015-0530-8 HEADACHE (RB HALKER, SECTION EDITOR) Dissecting the Association Between Migraine and Stroke Andrea M. Harriott & Kevin M. Barrett # Springer Science+Business Media New York 2015 Abstract Migraine is a common disabling neurological dis- Introduction order resulting from excessive cortical excitation and trigeminovascular afferent sensitization. In addition to aber- Migraine is a common neurological condition characterized rant neuronal processing, migraineurs are also at significant by recurrent unilateral headaches of moderate to severe inten- risk of vascular disease. Consequently, the impact of mi- sity lasting 4–72 h in duration. The pain is often pulsatile in graine extends well beyond the ictal headache and includes quality and associated with nausea, vomiting, and sensitivity a well-documented association with acute ischemic stroke, to light and sound. Migraines can be heralded by visual aura in particularly in young women with a history of migraine with addition to other somatosensory, vestibular, motor, and aura. The association between migraine and stroke has been speech-related disturbances [1–4]. Migraine impacts 15 to acknowledged for 40 years or more. However, examining 20 % of the general adult population, including approximately the pathobiology of this association has become a more 17 to 25 % of women and 6 to 10% of men [5–7]. Starting as recent and critically important undertaking. The diversity early as 12 years of age, the highest prevalence of migraine is of mechanisms underlying the association between migraine amongst persons 20 to 50 years of age [6, 8]. The social, and stroke likely reflects the heterogenous nature of this economic, and psychological burden of migraine alone can disorder.