Dissecting the Association Between Migraine and Stroke
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibromyalgia and Chronic Fatigue
Fibromyalgia and Chronic Fatigue Paul G. Swingle, Ph.D., R. Psych. My previous radio program and my present webcast program are both entitled “It’s all in your head!” How I came up with this title was listening to the discouraged patients that I routinely treated, and still treat, who have been told, by the doctors they have consulted, that the pain, discomfort, distress, debilitating fatigue, sleep disturbance and depression that they endure is “all in your head.” What this remark is meant to imply, of course, is that there is no physical cause of the condition and that the symptoms are manifestations of psychological factors. My response to these patients is that “of course it is in your head, where else would it be?” My remark however is not disparaging but rather indicates the actual location of many of the patients symptoms. The brain controls everything so all symptoms are associated with brain activity. The remarkable effectiveness of neurotherapy for the treatment of the symptoms associated with fibromyalgia and chronic fatigue is due to the fact that the brain functioning associated with the symptoms is treated. Although this derogatory sentiment about fibromyalgia patients was widespread among health care providers in the past, it is remarkable to me that it still persists with many doctors to this day. A few days ago I was surfing the internet health news feeds and was dismayed to find an item entitled “Fibromyalgia: A mental illness?” In this item was a quote from a Canadian neurologist stating that fibromyalgia was a term waiting for a definition. -

Stand up to Chronic Migraine with Botox®
#1 PRESCRIBED BRANDED TREATMENT FOR CHRONIC MIGRAINE* Chronic Migraine DON’T LIE DOWN BOTOX ® STAND UP TO Prevention CHRONIC MIGRAINE® WITH BOTOX Treatment Experience Treatment *Truven Health MarketScan Data, October 2010-April 2017. Prevent Headaches and Migraines Before They Even Start BOTOX ® BOTOX® prevents on average 8 to 9 headache days and migraine/probable migraine days a month (vs 6 to 7 for placebo) Savings Program For adults with Chronic Migraine, 15 or more headache days a month, each lasting 4 hours or more. BOTOX® is not approved for adults with migraine who have 14 or fewer headache days a month. Indication • Spread of toxin effects. The effect of botulinum toxin may affect areas BOTOX® is a prescription medicine that is injected to prevent headaches in adults away from the injection site and cause serious symptoms including: loss of with chronic migraine who have 15 or more days each month with headache strength and all-over muscle weakness, double vision, blurred vision and lasting 4 or more hours each day in people 18 years or older. drooping eyelids, hoarseness or change or loss of voice, trouble saying words It is not known whether BOTOX® is safe or effective to prevent headaches clearly, loss of bladder control, trouble breathing, trouble swallowing. in patients with migraine who have 14 or fewer headache days each month There has not been a confirmed serious case of spread of toxin effect away from (episodic migraine). the injection site when BOTOX® has been used at the recommended dose to IMPORTANT SAFETY INFORMATION treat chronic migraine. Resources BOTOX® may cause serious side effects that can be life threatening. -

SOS – Save Our Shoulders: a Guide for Polio Survivors
1 • Save Our Shoulders: A Guide for Polio Survivors A Guide for Polio Survivors S.O.S. Save Our Shoulders: A Guide for Polio Survivors by Jennifer Kuehl, MPT Roberta Costello, MSN, RN Janet Wechsler, PT Funding for the production of this manual was made possible by: The National Institute for Disability and Rehabilitation Research Grant #H133A000101 and The U.S. Department of the Army Grant #DAMD17-00-1-0533 Investigators: Mary Klein, PhD Mary Ann Keenan, MD Alberto Esquenazi, MD Acknowledgements We gratefully acknowledge the contributions and input provided from all of those who participated in our research. The time and effort of our participants was instrumental in the creation of this manual. Jennifer Kuehl, MPT Moss Rehabilitation Research Institute, Philadelphia Roberta Costello, MSN, RN Moss Rehabilitation Research Institute, Philadelphia Janet Wechsler, PT Moss Rehabilitation Research Institute, Philadelphia Mary Klein, PhD Moss Rehabilitation Research Institute, Philadelphia Mary Ann Keenan, MD University of Pennsylvania, Philadelphia Alberto Esquenazi, MD MossRehab Hospital, Philadelphia Cover and manual design by Ron Kalstein, MEd Albert Einstein Medical Center, Philadelphia Table of Contents 1. Introduction . .5 2. General Information About the Shoulder . .6 3. Facts About Shoulder Problems . .8 4. Treatment Options . .13 5. About Exercise . .16 6. Stretching Exercises . .19 7. Cane Stretches . .22 8. Strengthening Exercises . .25 9. Tips to Avoid Shoulder Problems . .29 10. Conclusion . .31 11. Resources . .31 The information contained within this manual is for reference only and is not a substitute for professional medical advice. Before beginning any exercise program consult your physician. Save Our Shoulders: A Guide for Polio Survivors • 4 Introduction Many polio survivors report new symptoms as they age. -

Vascular Dementia: an Overview of Current Challenges and Gaps
Stroke and Dementia: what research tells us UK Stroke Assembly 2016 JM Wardlaw University of Edinburgh NHS Lothian www.strokeassembly.org.uk #strokeassembly Definitions Stroke Dementia Sudden onset focal A syndrome where there neurological deficit which is deterioration in last more than 24 hours memory, thinking, attributable to a vascular behaviour such that the defect and has no other person is no longer able identifiable cause to perform everyday activities by themselves www.strokeassembly.org.uk #strokeassembly Stroke – the importance • Stroke, 16.9million new strokes per year, – A third are disabled and dependent – 2nd commonest cause of death – Commonest cause of dependency in adults Infarct, or ischaemic stroke Haemorrhagic stroke www.strokeassembly.org.uk #strokeassembly Cerebrovascular disease – the importance • ‘Silent’ cerebrovascular disease, even bigger issue – Up to 45% of 35.6million dementias – Cognitive impairment, mobility problems Normal White matter disease www.strokeassembly.org.uk #strokeassembly Dementia • 47.5 million people have dementia world wide • 7.7million new cases per year • Affects different people in different ways • Major cause of dependency and disability amongst older people • Alzheimer’s disease commonest cause – 60-70% of cases • Vascular dementia 2nd commonest – 25-45% of all cases www.strokeassembly.org.uk #strokeassembly Dementia: historical perspective 1800’s – Blood vessel diseases 1900’s – Alzheimers disease - dominant 1970’s – ‘Multi-infarct dementia’ – vascular 1990’s–2000’s - ‘Vascular -

The Migraine-Epilepsy Syndrome
medigraphic Artemisaen línea Arch Neurocien (Mex) Vol 11, No. 4: 282-287, 2006 The Migraine- Epilepsy Syndrome Arch Neurocien (Mex) Vol. 11, No. 4: 282-287, 2006 Artículo de revisión ©INNN, 2006 de caso The migraine-epilepsy syndrome Enrique Otero Siliceo†, Fernando Zermeño EL SINDROME MIGRAÑA-EPILEPSIA represent a neural exitation. Since that the glutamate has in important rol in both patologys depending of the part of the brain more affected the symptoms might RESUMEN vary from visual to abdominal phemomena. La migraña y la epilepsia tienen varios puntos en común Key words: migraine epilepsy, EEG abnormalities, sintomática clínica y genéticamente lo que ha sido glutamate, diagnosis. postulado por más de cien años. El fenómeno referido como migraña-epilepsia sugiere que exista una he first steps of a practical, approach by patofisiología común. El síndrome de migraña o physicians in recognizing and treating neuro- epilepsia tiene fenómenos comunes de dolor adominal T logic diseases are to recognithat there are jaqueca anormalidades del EE y respuesta a droga various overlaps between migraine and epilepsy. antiepilépticas. En ocasiones el paciente puede tener Epileptic seizures and classic migraine episodes may un ataque migrañoso o una convulsión o en otras occur in the same patient. Migraine and epilepsy share ambas. La comorbilidad puede explicarse por estados several genetic, clinical, evolutive and neurophysio- de hiperrexcitabilidad neural. Alteraciones electroen- logic features. A relationship between epilepsy and cefalográficas son comunes en estos estados. En migraine has been postulated for over a hundred years apariencia el glutamato tiene un papel importante tanto and the syndrome of Migraine-Epilepsy illustrates this en la migraña como en la epilepsia. -

Fatigue After Stroke
SPECIAL REPORT Fatigue after stroke PJ Tyrrell Fatigue is a common symptom after stroke. It is not invariably related to stroke severity & DG Smithard† and can occur in the absence of depression. It is one of the most troublesome symptoms for †Author for correspondence many patients and yet nothing is known of its causation. There are no specific treatments. William Harvey Hospital, Richard Steven’s Ward, This article assesses the available literature in the context of what is known about fatigue Ashford, Kent, in other disorders. Post-stroke fatigue may be a manifestation of sickness behavior, TN24 0LZ, UK mediated through the central effects of the cytokine interleukin-1, perhaps via effects on Tel.: +44 123 361 6214 Fax: +44 123 361 6662 glutamate neurotransmission. Possible therapeutic strategies are discussed which might be [email protected] a logical basis from which to plan randomized control trials. Following stroke, approximately a third of common and disabling symptom of Parkinson’s patients die, a third recover and a third remain disease [7,8] and of systemic lupus [9]. More than significantly disabled. Even those who recover 90% of patients with poliomyelitis develop a physically may be left with significant emotional delayed syndrome of post-myelitis fatigue [10]. and psychologic dysfunction – including anxi- Fatigue is the most prevalent symptom of ety, readjustment reactions and depression. One patients with cancer who receive radiation, cyto- common but often overlooked symptom is toxic or other therapies [11], and it may persist fatigue. This may occur soon or late after stroke, for years after the cessation of treatment [12]. -

Journal of Neurological Disorders DOI: 10.4172/2329-6895.1000275 ISSN: 2329-6895
olog eur ica N l D f i o s l o a r n d r e u r s o J Derakhshan, J Neurol Disord 2016, 4:4 Journal of Neurological Disorders DOI: 10.4172/2329-6895.1000275 ISSN: 2329-6895 Research Article Open Access Successful Opioid Monotherapy in Migralepsy: A Case Series Iraj Derakhshan* Department of Neurology, Case Western Reserve and Cincinnati Universities, Ohio, USA *Corresponding author: Iraj Derakhshan, Associate Professor, Department of Neurology, Case Western Reserve and Cincinnati Universities, Ohio, 205 Cyrus Drive, Charleston West Virginia, 25314, USA, Tel: 304 345 5174; E-mail: [email protected] Rec date: June 10, 2016; Acc date: July 06, 2016; Pub date: July 10, 2016 Copyright: © 2016 Derakhshan I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Background: There is a consensus that migraine and epilepsy are comorbid conditions. The novel concept explored and developed in this case series is that of the primacy of headaches in generating seizures in those patients suffering from migraine-triggered epilepsy (i.e., migralepsy). As demonstrated in the five cases descried here, much like the effect of ketogenic-diet on migraine-triggered epilepsy, once the migraine headaches were completely suppressed after adopting daily scheduled opioid therapy the seizures stopped from occurring, but they returned with the recurrence of the migraines once the patients had stopped their daily opiate regimen for any reason. Clinical implications: The above pharmacological scenario is reminiscent of a similar but naturalistic course of events as described in reports concerning the salutary effects of ketogenic diet, or restoration of sleep, in cases of migraine-triggered epilepsy. -

Migraine Mimics
ISSN 0017-8748 Headache doi: 10.1111/head.12518 © 2015 American Headache Society Published by Wiley Periodicals, Inc. Expert Opinion Migraine Mimics Randolph W. Evans, MD The symptoms of migraine are non-specific and can be present in many other primary and secondary headache disorders, which are reviewed. Even experienced headache specialists may be challenged at times when diagnosing what appears to be first or worst, new type, migraine status, and chronic migraine. Key words: migraine, migraine mimic, symptomatic migraine, hemicrania continua (Headache 2015;55:313-322) The symptoms of migraine are non-specific and She had seen 2 headache specialists previously. can be present in many other primary and secondary She had been tried on sumatriptan p.o. and subcuta- headache disorders.1,2 Even experienced headache neously, diclofenac powder, ketorolac oral and intra- specialists may be challenged at times when diagnos- muscular, dihydroergotamine nasal spray, and had an ing what appears to be first or worst, new type, occipital nerve block without benefit. Gabapentin migraine status, and chronic migraine. Another diag- and pregabalin did not help. She was placed on indo- nosis may be responsible when physicians use the term methacin 75 mg sustained release once a day for 8 “atypical migraine.” days without benefit. Prednisone 60 mg daily for 10 days did not help.An intravenous dihydroergotamine CASE HISTORIES regimen for 5 days did not help. Case 1.—This 48-year-old woman was seen for a A magnetic resonance imaging (MRI) and mag- third opinion with a 20-year history of only menstrual netic resonance angiogram (MRA) of the brain and headaches always preceded by a visual aura followed cervical spine and magnetic resonance venogram by a generalized throbbing with an intensity of 5–6/10 (MRV) of the brain were negative. -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -

Decreased Risk of Dementia in Migraine Patients with Traditional Chinese Medicine Use: a Population-Based Cohort Study
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 45), pp: 79680-79692 Clinical Research Paper Decreased risk of dementia in migraine patients with traditional Chinese medicine use: a population-based cohort study Chun-Ting Liu1,*, Bei-Yu Wu1,*, Yu-Chiang Hung1,2,*, Lin-Yi Wang3, Yan-Yuh Lee3, Tsu-Kung Lin4, Pao-Yen Lin5, Wu-Fu Chen6, Jen-Huai Chiang7,8, Sheng-Feng Hsu9,10 and Wen-Long Hu1,11,12,* 1Department of Chinese Medicine, Kaohsiung Chang Gung Memorial Hospital and School of Traditional Chinese Medicine, Chang Gung University College of Medicine, Kaohsiung, Taiwan 2School of Chinese Medicine for Post Baccalaureate, I-Shou University, Kaohsiung, Taiwan 3Department of Physical Medicine and Rehabilitation, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan 4Department of Neurology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan 5Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan 6Department of Neurosurgery, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan 7Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan 8College of Medicine, China Medical University, Taichung, Taiwan 9Graduate Institute of Acupuncture Science, China Medical University, Taichung, Taiwan 10Department of Chinese Medicine, China Medical University Hospital, Taipei Branch, Taipei, Taiwan 11Kaohsiung Medical University College of Medicine, Kaohsiung, Taiwan 12Fooyin University College of Nursing, Kaohsiung, Taiwan *These authors contributed equally to this work Correspondence to: Wen-Long Hu, email: [email protected] Keywords: dementia, migraine, pharmaco-epidemiology, national health insurance research database, Chinese herbal product Received: February 27, 2017 Accepted: June 28, 2017 Published: July 08, 2017 Copyright: Liu et al. -
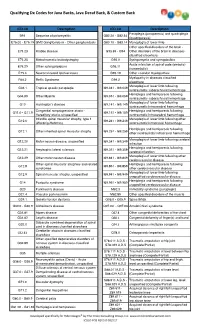
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -

Episodic Visual Snow Associated with Migraine Attacks
Letters RESEARCH LETTER Discussion | Three patients report episodes of VS exclusively at the beginning or during migraine attacks. The description was Episodic Visual Snow Associated identical and matched the definition of VS in VSS except for With Migraine Attacks not being continuous.1,2 In the syndrome-defining study,1 only Visual snow syndrome (VSS) is a debilitating disorder charac- patients with continuous VS were included, impeding the iden- terized by continuous visual snow (VS), ie, tiny flickering dots tification of an episodic form. Based on the present case se- in the entire visual field resembling the view of a badly tuned ries, we propose to distinguish between VSS, a debilitating dis- analog television (Figure), plus additional visual symptoms, order characterized by continuous VS and additional visual such as photophobia and palinopsia. There is a high comor- symptoms persisting over years, and eVS, an uncommon self- 1 bidity with migraine and migraine aura. To our knowledge, limiting symptom during migraine attacks. this is the first report of patients with an episodic form of VS The relationship between migraine and VSS is still (eVS), strictly co-occurring with migraine attacks. unresolved.3 Although the severity of VS in VSS does not fluc- tuate in parallel to the migraine cycle,1 the strict co-occurrence Methods | Between January 2016 and December 2017, we saw of eVS and migraine reported here epitomizes a close proxim- 3 patients with eVS and 1934 patients with migraine at our ter- ity.This is in agreement with the clinical picture of migraine being tiary outpatient headache center.