Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibromyalgia and Chronic Fatigue
Fibromyalgia and Chronic Fatigue Paul G. Swingle, Ph.D., R. Psych. My previous radio program and my present webcast program are both entitled “It’s all in your head!” How I came up with this title was listening to the discouraged patients that I routinely treated, and still treat, who have been told, by the doctors they have consulted, that the pain, discomfort, distress, debilitating fatigue, sleep disturbance and depression that they endure is “all in your head.” What this remark is meant to imply, of course, is that there is no physical cause of the condition and that the symptoms are manifestations of psychological factors. My response to these patients is that “of course it is in your head, where else would it be?” My remark however is not disparaging but rather indicates the actual location of many of the patients symptoms. The brain controls everything so all symptoms are associated with brain activity. The remarkable effectiveness of neurotherapy for the treatment of the symptoms associated with fibromyalgia and chronic fatigue is due to the fact that the brain functioning associated with the symptoms is treated. Although this derogatory sentiment about fibromyalgia patients was widespread among health care providers in the past, it is remarkable to me that it still persists with many doctors to this day. A few days ago I was surfing the internet health news feeds and was dismayed to find an item entitled “Fibromyalgia: A mental illness?” In this item was a quote from a Canadian neurologist stating that fibromyalgia was a term waiting for a definition. -

Exosome TDP-43 Profile in Canine Model of ALS: a Preliminary Study in Developing Surrogate Biomarker
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.17.448876; this version posted June 18, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Exosome TDP-43 profile in canine model of ALS: A preliminary study in developing surrogate biomarker Penelope Pfeiffer 1, Joan R. Coates 2, Andrew Kennedy3, Kyleigh Getchell3, Edina Kosa3, Abdulbaki Agbas 3, 4* 1 Mount Sinai Hospital, Chicago IL; [email protected] 2 University of Missouri-Columbia, MO; [email protected] 3 Kansas City University, Kansas City MO; [email protected]; [email protected]; [email protected]; [email protected] 4 Heartland Center for Mitochondrial Medicine, Kansas City KS * Correspondence: [email protected]; Tel.: +1 816-654-7614 Abstract: Blood-based biomarkers are much-needed diagnostic and prognostic tools for ALS. Canine degenerative myelopathy (DM) is recognized animal disease model to study the biology of human ALS. Serum derived exosomes are potential carrier that transport intercellular hormone-like messengers, together with their stability as carrier of proteins and RNA, make them ideal as biomarkers for a variety of diseases and biological processes. We study exosomal TDP-43 pattern as a surrogate biomarker that reflects biochemical changes in central nervous system. We isolated exosomes from canine serum using commercial exosome isolation reagents. TDP-43 and SOD1 profile in spinal cord homogenate lysate and that of serum-derived exosomes were found elevated in dogs with DM. -

SOS – Save Our Shoulders: a Guide for Polio Survivors
1 • Save Our Shoulders: A Guide for Polio Survivors A Guide for Polio Survivors S.O.S. Save Our Shoulders: A Guide for Polio Survivors by Jennifer Kuehl, MPT Roberta Costello, MSN, RN Janet Wechsler, PT Funding for the production of this manual was made possible by: The National Institute for Disability and Rehabilitation Research Grant #H133A000101 and The U.S. Department of the Army Grant #DAMD17-00-1-0533 Investigators: Mary Klein, PhD Mary Ann Keenan, MD Alberto Esquenazi, MD Acknowledgements We gratefully acknowledge the contributions and input provided from all of those who participated in our research. The time and effort of our participants was instrumental in the creation of this manual. Jennifer Kuehl, MPT Moss Rehabilitation Research Institute, Philadelphia Roberta Costello, MSN, RN Moss Rehabilitation Research Institute, Philadelphia Janet Wechsler, PT Moss Rehabilitation Research Institute, Philadelphia Mary Klein, PhD Moss Rehabilitation Research Institute, Philadelphia Mary Ann Keenan, MD University of Pennsylvania, Philadelphia Alberto Esquenazi, MD MossRehab Hospital, Philadelphia Cover and manual design by Ron Kalstein, MEd Albert Einstein Medical Center, Philadelphia Table of Contents 1. Introduction . .5 2. General Information About the Shoulder . .6 3. Facts About Shoulder Problems . .8 4. Treatment Options . .13 5. About Exercise . .16 6. Stretching Exercises . .19 7. Cane Stretches . .22 8. Strengthening Exercises . .25 9. Tips to Avoid Shoulder Problems . .29 10. Conclusion . .31 11. Resources . .31 The information contained within this manual is for reference only and is not a substitute for professional medical advice. Before beginning any exercise program consult your physician. Save Our Shoulders: A Guide for Polio Survivors • 4 Introduction Many polio survivors report new symptoms as they age. -

Vascular Dementia: an Overview of Current Challenges and Gaps
Stroke and Dementia: what research tells us UK Stroke Assembly 2016 JM Wardlaw University of Edinburgh NHS Lothian www.strokeassembly.org.uk #strokeassembly Definitions Stroke Dementia Sudden onset focal A syndrome where there neurological deficit which is deterioration in last more than 24 hours memory, thinking, attributable to a vascular behaviour such that the defect and has no other person is no longer able identifiable cause to perform everyday activities by themselves www.strokeassembly.org.uk #strokeassembly Stroke – the importance • Stroke, 16.9million new strokes per year, – A third are disabled and dependent – 2nd commonest cause of death – Commonest cause of dependency in adults Infarct, or ischaemic stroke Haemorrhagic stroke www.strokeassembly.org.uk #strokeassembly Cerebrovascular disease – the importance • ‘Silent’ cerebrovascular disease, even bigger issue – Up to 45% of 35.6million dementias – Cognitive impairment, mobility problems Normal White matter disease www.strokeassembly.org.uk #strokeassembly Dementia • 47.5 million people have dementia world wide • 7.7million new cases per year • Affects different people in different ways • Major cause of dependency and disability amongst older people • Alzheimer’s disease commonest cause – 60-70% of cases • Vascular dementia 2nd commonest – 25-45% of all cases www.strokeassembly.org.uk #strokeassembly Dementia: historical perspective 1800’s – Blood vessel diseases 1900’s – Alzheimers disease - dominant 1970’s – ‘Multi-infarct dementia’ – vascular 1990’s–2000’s - ‘Vascular -

What to Expect After Having a Subarachnoid Hemorrhage (SAH) Information for Patients and Families Table of Contents
What to expect after having a subarachnoid hemorrhage (SAH) Information for patients and families Table of contents What is a subarachnoid hemorrhage (SAH)? .......................................... 3 What are the signs that I may have had an SAH? .................................. 4 How did I get this aneurysm? ..................................................................... 4 Why do aneurysms need to be treated?.................................................... 4 What is an angiogram? .................................................................................. 5 How are aneurysms repaired? ..................................................................... 6 What are common complications after having an SAH? ..................... 8 What is vasospasm? ...................................................................................... 8 What is hydrocephalus? ............................................................................... 10 What is hyponatremia? ................................................................................ 12 What happens as I begin to get better? .................................................... 13 What can I expect after I leave the hospital? .......................................... 13 How will the SAH change my health? ........................................................ 14 Will the SAH cause any long-term effects? ............................................. 14 How will my emotions be affected? .......................................................... 15 When should -

Fatigue After Stroke
SPECIAL REPORT Fatigue after stroke PJ Tyrrell Fatigue is a common symptom after stroke. It is not invariably related to stroke severity & DG Smithard† and can occur in the absence of depression. It is one of the most troublesome symptoms for †Author for correspondence many patients and yet nothing is known of its causation. There are no specific treatments. William Harvey Hospital, Richard Steven’s Ward, This article assesses the available literature in the context of what is known about fatigue Ashford, Kent, in other disorders. Post-stroke fatigue may be a manifestation of sickness behavior, TN24 0LZ, UK mediated through the central effects of the cytokine interleukin-1, perhaps via effects on Tel.: +44 123 361 6214 Fax: +44 123 361 6662 glutamate neurotransmission. Possible therapeutic strategies are discussed which might be [email protected] a logical basis from which to plan randomized control trials. Following stroke, approximately a third of common and disabling symptom of Parkinson’s patients die, a third recover and a third remain disease [7,8] and of systemic lupus [9]. More than significantly disabled. Even those who recover 90% of patients with poliomyelitis develop a physically may be left with significant emotional delayed syndrome of post-myelitis fatigue [10]. and psychologic dysfunction – including anxi- Fatigue is the most prevalent symptom of ety, readjustment reactions and depression. One patients with cancer who receive radiation, cyto- common but often overlooked symptom is toxic or other therapies [11], and it may persist fatigue. This may occur soon or late after stroke, for years after the cessation of treatment [12]. -

What%Is%Epilepsy?%
What%is%Epilepsy?% Epilepsy(is(a(brain(disorder(in(which(a(person(has(repeated(seizures((convulsions)(over(time.(Seizures(are( episodes(of(disturbed(brain(activity(that(cause(changes(in(attention(or(behavior.( Causes( Epilepsy(occurs(when(permanent(changes(in(brain(tissue(cause(the(brain(to(be(too(excitable(or(jumpy.( The(brain(sends(out(abnormal(signals.(This(results(in(repeated,(unpredictable(seizures.((A(single(seizure( that(does(not(happen(again(is(not(epilepsy.)( Epilepsy(may(be(due(to(a(medical(condition(or(injury(that(affects(the(brain,(or(the(cause(may(be( unknown((idiopathic).( Common(causes(of(epilepsy(include:( •Stroke(or(transient(ischemic(attack((TIA)( •Dementia,(such(as(Alzheimer's(disease( •Traumatic(brain(injury( •Infections,(including(brain(abscess,(meningitis,(encephalitis,(and(AIDS( •Brain(problems(that(are(present(at(birth((congenital(brain(defect)( •Brain(injury(that(occurs(during(or(near(birth( •Metabolism(disorders(present(at(birth((such(as(phenylketonuria)( •Brain(tumor( •Abnormal(blood(vessels(in(the(brain( •Other(illness(that(damage(or(destroy(brain(tissue( •Use(of(certain(medications,(including(antidepressants,(tramadol,(cocaine,(and(amphetamines( Epilepsy(seizures(usually(begin(between(ages(5(and(20,(but(they(can(happen(at(any(age.(There(may(be(a( family(history(of(seizures(or(epilepsy.( Symptoms( Symptoms(vary(from(person(to(person.(Some(people(may(have(simple(staring(spells,(while(others(have( violent(shaking(and(loss(of(alertness.(The(type(of(seizure(depends(on(the(part(of(the(brain(affected(and( cause(of(epilepsy.( -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -

The Good and the Bad of CRISPR/Cas9 Genome Editing
Replicating human disease in rodents: the good and the bad of CRISPR/Cas9 genome editing Guillaume Pavlovic, PhD Head of Unit at PHENOMIN ‐ Institut Clinique de la Souris email: [email protected] CMC Strategy Forum Europe linkedIn: http://bit.ly/2VMLoJ9 Understanding Human disease with rodents: three challenges phenotypes of protein Some failure of mice and other coding genes in Mouse model organisms studies to be replicated or translated to humans Multiple phenotypes No known Knowledge Translation phenotype on disease from rodent One genes to Human phenotype Probability of success Reproducibility of preclinical studies https://www.mousephenotype.org/ 50% of preclinical data are irreproducible CRISPR/Cas9 genome editing in rodents THE GOOD What new possibilities does CRISPR open to better mimic human diseases ? – The genetic context (background) is not adapted – The designed mutation does not mimic the Human pathology With CRISPR/Cas9 new types of mutations can be easily engineered THE BAD There is more than off targets ! Understanding CRISPR/Cas9 genome editing to reduce lack of reproducibility – You are facing experimental variability, poor experimental design or bad reproducibility why do the results discovered using in vivo models sometimes fail to translate to human disease? Plenty of literature showing that the inbred genetic background has an effect on the phenotype Cerebellar phenotype of En1Hd/Hd mutants on 129/Sv and C57BL/6J backgrounds. deletion of the cerebellum 129/Sv—En1Hd/Hd C57BL/6J—En1Hd/Hd Bilovocky et al. J. Neurosci. 2003 why do the results discovered using in vivo models sometimes fail to translate to human disease? Plenty of literature showing that the inbred genetic background has an effect on the phenotype genetic background limits generalizability of genotype-phenotype relationships Sittig et al. -

Current and Emerging Treatment Options for Endometriosis
Expert Opinion on Pharmacotherapy ISSN: 1465-6566 (Print) 1744-7666 (Online) Journal homepage: http://www.tandfonline.com/loi/ieop20 Current and emerging treatment options for endometriosis Simone Ferrero, Giulio Evangelisti & Fabio Barra To cite this article: Simone Ferrero, Giulio Evangelisti & Fabio Barra (2018): Current and emerging treatment options for endometriosis, Expert Opinion on Pharmacotherapy, DOI: 10.1080/14656566.2018.1494154 To link to this article: https://doi.org/10.1080/14656566.2018.1494154 Published online: 05 Jul 2018. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ieop20 EXPERT OPINION ON PHARMACOTHERAPY https://doi.org/10.1080/14656566.2018.1494154 REVIEW Current and emerging treatment options for endometriosis Simone Ferrero a,b, Giulio Evangelistia,b and Fabio Barra a,b aAcademic Unit of Obstetrics and Gynecology, Ospedale Policlinico San Martino, Genoa, Italy; bDepartment of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DiNOGMI), University of Genoa, Genoa, Italy ABSTRACT ARTICLE HISTORY Introduction: Pharmacotherapy has a pivotal role in the management of endometriosis with long-term Received 8 June 2018 treatments balancing clinical efficacy (control of pain symptoms and prevention of recurrence of the Accepted 25 June 2018 disease after surgery) with an acceptable safety profile. Treatment choice is based on several factors KEYWORDS including age and patient preference, reproductive plans, intensity of pain, severity of disease and Endometriosis; combined incidence of adverse effects. oral contraceptives; Areas covered: The aim of this review is to provide the reader with a complete overview of drugs that progestins; gonadotropin- are currently available or are under investigation for the treatment of endometriosis highlighting on- releasing hormone agonist; going clinical trials. -
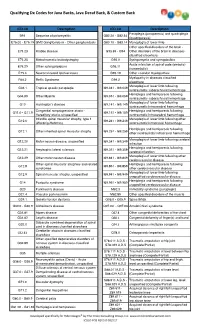
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -

Brain Injury and Opioid Overdose
Brain Injury and Opioid Overdose: Acquired Brain Injury is damage to the brain 2.8 million brain injury related occurring after birth and is not related to congenital or degenerative disease. This includes anoxia and hospital stays/deaths in 2013 hypoxia, impairment (lack of oxygen), a condition consistent with drug overdose. 70-80% of hospitalized patients are discharged with an opioid Rx Opioid Use Disorder, as defined in DSM 5, is a problematic pattern of opioid use leading to clinically significant impairment, manifested by meaningful risk 63,000+ drug overdose-related factors occurring within a 12-month period. deaths in 2016 Overdose is injury to the body (poisoning) that happens when a drug is taken in excessive amounts “As the number of drug overdoses continues to rise, and can be fatal. Opioid overdose induces respiratory doctors are struggling to cope with the increasing number depression that can lead to anoxic or hypoxic brain of patients facing irreversible brain damage and other long injury. term health issues.” Substance Use and Misuse is: The frontal lobe is • Often a contributing factor to brain injury. History of highly susceptible abuse/misuse is common among individuals who to brain oxygen have sustained a brain injury. loss, and damage • Likely to increase for individuals who have misused leads to potential substances prior to and post-injury. loss of executive Acute or chronic pain is a common result after brain function. injury due to: • Headaches, back or neck pain and other musculo- Sources: Stojanovic et al 2016; Melton, C. Nov. 15,2017; Devi E. skeletal conditions commonly reported by veterans Nampiaparampil, M.D., 2008; Seal K.H., Bertenthal D., Barnes D.E., et al 2017; with a history of brain injury.