The Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (Veritas) Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibromyalgia and Chronic Fatigue
Fibromyalgia and Chronic Fatigue Paul G. Swingle, Ph.D., R. Psych. My previous radio program and my present webcast program are both entitled “It’s all in your head!” How I came up with this title was listening to the discouraged patients that I routinely treated, and still treat, who have been told, by the doctors they have consulted, that the pain, discomfort, distress, debilitating fatigue, sleep disturbance and depression that they endure is “all in your head.” What this remark is meant to imply, of course, is that there is no physical cause of the condition and that the symptoms are manifestations of psychological factors. My response to these patients is that “of course it is in your head, where else would it be?” My remark however is not disparaging but rather indicates the actual location of many of the patients symptoms. The brain controls everything so all symptoms are associated with brain activity. The remarkable effectiveness of neurotherapy for the treatment of the symptoms associated with fibromyalgia and chronic fatigue is due to the fact that the brain functioning associated with the symptoms is treated. Although this derogatory sentiment about fibromyalgia patients was widespread among health care providers in the past, it is remarkable to me that it still persists with many doctors to this day. A few days ago I was surfing the internet health news feeds and was dismayed to find an item entitled “Fibromyalgia: A mental illness?” In this item was a quote from a Canadian neurologist stating that fibromyalgia was a term waiting for a definition. -

SOS – Save Our Shoulders: a Guide for Polio Survivors
1 • Save Our Shoulders: A Guide for Polio Survivors A Guide for Polio Survivors S.O.S. Save Our Shoulders: A Guide for Polio Survivors by Jennifer Kuehl, MPT Roberta Costello, MSN, RN Janet Wechsler, PT Funding for the production of this manual was made possible by: The National Institute for Disability and Rehabilitation Research Grant #H133A000101 and The U.S. Department of the Army Grant #DAMD17-00-1-0533 Investigators: Mary Klein, PhD Mary Ann Keenan, MD Alberto Esquenazi, MD Acknowledgements We gratefully acknowledge the contributions and input provided from all of those who participated in our research. The time and effort of our participants was instrumental in the creation of this manual. Jennifer Kuehl, MPT Moss Rehabilitation Research Institute, Philadelphia Roberta Costello, MSN, RN Moss Rehabilitation Research Institute, Philadelphia Janet Wechsler, PT Moss Rehabilitation Research Institute, Philadelphia Mary Klein, PhD Moss Rehabilitation Research Institute, Philadelphia Mary Ann Keenan, MD University of Pennsylvania, Philadelphia Alberto Esquenazi, MD MossRehab Hospital, Philadelphia Cover and manual design by Ron Kalstein, MEd Albert Einstein Medical Center, Philadelphia Table of Contents 1. Introduction . .5 2. General Information About the Shoulder . .6 3. Facts About Shoulder Problems . .8 4. Treatment Options . .13 5. About Exercise . .16 6. Stretching Exercises . .19 7. Cane Stretches . .22 8. Strengthening Exercises . .25 9. Tips to Avoid Shoulder Problems . .29 10. Conclusion . .31 11. Resources . .31 The information contained within this manual is for reference only and is not a substitute for professional medical advice. Before beginning any exercise program consult your physician. Save Our Shoulders: A Guide for Polio Survivors • 4 Introduction Many polio survivors report new symptoms as they age. -

Vascular Dementia: an Overview of Current Challenges and Gaps
Stroke and Dementia: what research tells us UK Stroke Assembly 2016 JM Wardlaw University of Edinburgh NHS Lothian www.strokeassembly.org.uk #strokeassembly Definitions Stroke Dementia Sudden onset focal A syndrome where there neurological deficit which is deterioration in last more than 24 hours memory, thinking, attributable to a vascular behaviour such that the defect and has no other person is no longer able identifiable cause to perform everyday activities by themselves www.strokeassembly.org.uk #strokeassembly Stroke – the importance • Stroke, 16.9million new strokes per year, – A third are disabled and dependent – 2nd commonest cause of death – Commonest cause of dependency in adults Infarct, or ischaemic stroke Haemorrhagic stroke www.strokeassembly.org.uk #strokeassembly Cerebrovascular disease – the importance • ‘Silent’ cerebrovascular disease, even bigger issue – Up to 45% of 35.6million dementias – Cognitive impairment, mobility problems Normal White matter disease www.strokeassembly.org.uk #strokeassembly Dementia • 47.5 million people have dementia world wide • 7.7million new cases per year • Affects different people in different ways • Major cause of dependency and disability amongst older people • Alzheimer’s disease commonest cause – 60-70% of cases • Vascular dementia 2nd commonest – 25-45% of all cases www.strokeassembly.org.uk #strokeassembly Dementia: historical perspective 1800’s – Blood vessel diseases 1900’s – Alzheimers disease - dominant 1970’s – ‘Multi-infarct dementia’ – vascular 1990’s–2000’s - ‘Vascular -

Fatigue After Stroke
SPECIAL REPORT Fatigue after stroke PJ Tyrrell Fatigue is a common symptom after stroke. It is not invariably related to stroke severity & DG Smithard† and can occur in the absence of depression. It is one of the most troublesome symptoms for †Author for correspondence many patients and yet nothing is known of its causation. There are no specific treatments. William Harvey Hospital, Richard Steven’s Ward, This article assesses the available literature in the context of what is known about fatigue Ashford, Kent, in other disorders. Post-stroke fatigue may be a manifestation of sickness behavior, TN24 0LZ, UK mediated through the central effects of the cytokine interleukin-1, perhaps via effects on Tel.: +44 123 361 6214 Fax: +44 123 361 6662 glutamate neurotransmission. Possible therapeutic strategies are discussed which might be [email protected] a logical basis from which to plan randomized control trials. Following stroke, approximately a third of common and disabling symptom of Parkinson’s patients die, a third recover and a third remain disease [7,8] and of systemic lupus [9]. More than significantly disabled. Even those who recover 90% of patients with poliomyelitis develop a physically may be left with significant emotional delayed syndrome of post-myelitis fatigue [10]. and psychologic dysfunction – including anxi- Fatigue is the most prevalent symptom of ety, readjustment reactions and depression. One patients with cancer who receive radiation, cyto- common but often overlooked symptom is toxic or other therapies [11], and it may persist fatigue. This may occur soon or late after stroke, for years after the cessation of treatment [12]. -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -
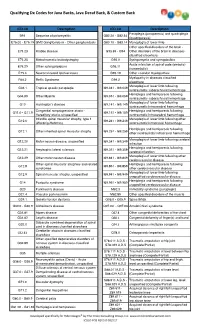
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -
Stroke: Learn the Warning Signs and Protect Yourself Strokes Are the Leading Cause of Major Disability in the U.S
stroke: learn the warning signs and protect yourself Strokes are the leading cause of major disability in the U.S. and the second leading cause of death worldwide. With all the emphasis placed on heart disease reaches us, they’ve already been to the hospital. The and cancer as the two leading causes of death in only thing left by then is active physical therapy.” America, people often forget what ranks as third. The answer is stroke, and it can have debilitating Dr. Ray says that is why he preaches religiously effects even if you survive. to patients about lowering blood pressure and cholesterol, healthy diet and regular exercise, and On average, someone has a stroke in the U.S. every keeping any pre-existing conditions in check through 45 seconds, which equals 700,000 strokes a year. regular checkups. He goes on to say that having a Of these, about 500,000 are first-time strokes. primary care physician is vital. Strokes are so pervasive that they are the leading cause of major disability in the “All adults should speak to their doctors U.S. and the second leading cause of death about diet and controlling their blood worldwide. pressure,” Dr. Ray says. “You want to actively prevent this before it happens.” A stroke occurs when blood flow to parts of the brain is restricted or impaired, There are two basic types of stroke. The first killing off brain cells. Those cells can never is ischemic stroke caused by blood clots or regenerate, which means the damage is other particles in the blood vessels leading permanent. -

Central Pain: Clinical and Physiological J Neurol Neurosurg Psychiatry: First Published As 10.1136/Jnnp.61.1.62 on 1 July 1996
626Journal ofNeurology, Neurosurgery, and Psychiatry 1996;61:62-69 Central pain: clinical and physiological J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.61.1.62 on 1 July 1996. Downloaded from characteristics David Bowsher Abstract spinothalamic pathway, its relays, or projec- Objectives-To study the clinical and tions may cause central pain,' and modern pathophysiological features of central radiological techniques have tended to con- pain due to damage to the CNS. firm this.9 '" Because of this, and of recent Methods-156 patients (mostly with considerations on pathophysiology,'5 '" the ischaemic strokes, some with infarct after condition is now known as "central poststroke subarachnoid haemorrhage and other pain (CPSP)"'.'7 Stroke is not the only condi- cerebral conditions; one with bulbar and tion causing such central pains of cerebral ori- others with spinal pathology) with central gin.' lxII 2' Furthermore, identical pains occur pain have been investigated clinically and after some spinal lesions.- 22 varying numbers instrumentally with respect to quantitative somatosensory perception thresholds and autonomic Patients function. Between 1983 and 1993, 156 patients have Results-Pain onset was immediate in a been referred to me, in whom a diagnosis of minority; and from a week or two up to central pain due to a cerebral or spinal lesion six years in > 60%. For those with supra- has been made. Of these, 112 (64 (57)%'Y spinal ischaemic lesions, the median age male) had a history of a stroke episode (cere- of onset was 59; dominant and non- brovascular accident, CVA patients), includ- dominant sides were equally affected. -

Epilepsy After Stroke
Stroke Helpline: 0303 3033 100 Website: stroke.org.uk Epilepsy after stroke In the first few weeks after a stroke some people have a seizure, and a small number go on to develop epilepsy – a tendency to have repeated seizures. These can usually be completely controlled with treatment. This factsheet explains what epilepsy is, the different types of seizures, and how epilepsy is diagnosed and treated. It also includes advice about coping with a seizure, and a glossary. Epilepsy is a tendency to have repeated What causes seizures? seizures – sometimes called ‘fits’ or ‘attacks’. It affects just under one per cent Cells in the brain communicate with one of people in the UK. Stroke is one of many another and with our muscles by passing conditions that can lead to epilepsy. electrical signals along nerve fibres. If you have epilepsy this electrical activity can Around five per cent of people who have a become disordered. A sudden abnormal stroke will have a seizure within the following burst of electrical activity in the brain can few weeks. These are known as acute or lead to a seizure. onset seizures and normally happen within 24 hours of the stroke. You are more likely There are over 40 different types of seizures to have one if you have had a severe stroke, ranging from tingling sensations or ‘going a stroke caused by bleeding in the brain (a blank’ for a few seconds, to shaking and haemorrhagic stroke), or a stroke involving losing consciousness. the part of the brain called the cerebral cortex. If you have an onset seizure, it This can mean that epilepsy is sometimes does not necessarily mean you have or will confused with other conditions, including develop epilepsy. -

Acute Ischemic Stroke in Young Adults with Tuberculous Meningitis Liming Zhang1, Xiaoyu Zhang2, Huaqiang Li1,3, Gang Chen1* and Meijia Zhu2*
Zhang et al. BMC Infectious Diseases (2019) 19:362 https://doi.org/10.1186/s12879-019-4004-5 RESEARCHARTICLE Open Access Acute ischemic stroke in young adults with tuberculous meningitis Liming Zhang1, Xiaoyu Zhang2, Huaqiang Li1,3, Gang Chen1* and Meijia Zhu2* Abstract Background: Ischemic stroke is a common complication in patients with tuberculous meningitis (TBM), which is associated with poor clinical outcome. However, risk factors of stroke in TBM patients were not fully understood, especially in those young adults. Therefore, the aim of our study was to identify risk factors for acute ischemic stroke in young adults with TBM. Methods: TBM patients (18 to 50 years) without cerebral vascular risk factors were prospective recruited between Feb 2014 and Dec 2017. Patients were defined as stroke group and non-stroke group by brain magnetic resonance imaging (MRI). Demographic characteristics, clinical presentations, cerebrospinal fluid (CSF) examination, basal meningeal enhancement, hydrocephalus, tuberculoma and clinical outcome were compared between two groups. Binary logistic regression was performed to determine risk factors for acute ischemic stroke in young TBM patients. Results: Fifty-two patients with TBM were included and 12 (23.1%) patients were in stroke group. Patients in stroke group were older. Clinical presentations were comparable between two groups except headache was more common in TBM patients with stroke. In CSF examination, TBM patients with stroke had higher CSF white blood cell. By MRI, patients in stroke group were more likely to have basal meningeal enhancement but less likely to present tuberculoma. Compared to non-stroke group, patients in stroke group had worse short-term clinical outcome. -

Bacterial Meningitis and Neurological Complications in Adults
OCUSED EVIEW Parunyou J. et al. Bacterial meningitis F R Bacterial meningitis and neurological complications in adults Parunyou Julayanont MD, Doungporn Ruthirago MD, John C. DeToledo MD ABSTRACT Bacterial meningitis is a leading cause of death from infectious disease worldwide. The neurological complications secondary to bacterial meningitis contribute to the high mortality rate and to disability among the survivors. Cerebrovascular complications, including infarc- tion and hemorrhage, are common. Inflammation and increased pressure in the subarach- noid space result in cranial neuropathy. Seizures occur in either the acute or delayed phase after the infection and require early detection and treatment. Spreading of infection to other intracranial structures, including the subdural space, brain parenchyma, and ventricles, in- creases morbidity and mortality in survivors. Infection can also spread to the spinal canal causing spinal cord abscess, epidural abscess, polyradiculitis, and spinal cord infarction secondary to vasculitis of the spinal artery. Hypothalamic-pituitary dysfunction is also an un- common complication after bacterial meningitis. Damage to cerebral structures contributes to cognitive and neuropsychiatric problems. Being aware of these complications leads to early detection and treatment and improves mortality and outcomes in patients with bacte- rial meningitis. Key words: meningitis; meningitis, bacterial; central nervous system bacterial infection; nervous system diseases INTRODUCTION prove recovery and outcomes. Bacterial meningitis is a leading cause of death In this article, we present a case of bacterial men- from infectious disease worldwide. Despite the avail- ingitis complicated by an unusual number of neuro- ability of increasingly effective antibiotics and inten- logical complications that occurred in spite of a timely sive neurological care, the overall mortality remains diagnosis, adequate treatment, and intensive neuro- high, with 17-34% of the survivors having unfavorable logical monitoring. -
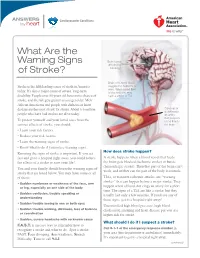
What Are the Warning Signs of Stroke? (PDF)
ANSWERS Cardiovascular Conditions by heart What Are the Warning Signs Brain tissue affected by of Stroke? blockage Brain cells need blood, Stroke is the fifth leading cause of death in America oxygen and nutrients to work. When blood flow today. It’s also a major cause of severe, long-term is blocked, you may disability. People over 55 years old have more chance of have a stroke or TIA. stroke, and the risk gets greater as you get older. Men, African Americans and people with diabetes or heart disease are the most at risk for stroke. About 6.6 million Close-up of a clot inside people who have had strokes are alive today. an artery that provides To protect yourself and your loved ones from the blood flow to serious effects of stroke, you should: the brain • Learn your risk factors. • Reduce your risk factors. • Learn the warning signs of stroke. • Know what to do if you notice warning signs. How does stroke happen? Knowing the signs of stroke is important. If you act fast and go to a hospital right away, you could reduce A stroke happens when a blood vessel that feeds the effects of a stroke or save your life! the brain gets blocked (ischemic stroke) or bursts (hemorrhagic stroke). Then that part of the brain can’t You and your family should learn the warning signs of work, and neither can the part of the body it controls. stroke that are listed below. You may have some or all of them: TIAs, or transient ischemic attacks, are “warning strokes” that can happen before a major stroke.