Uterine Preservation After Vaginal Delivery with Manual Extraction of Focal Placenta Accreta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mammalogy Lecture 12
Mammalogy Lecture 11 - Reproduction I: General Patterns I. Obviously, reproduction is a very important aspect of biology and whole courses are dedicated to it. It’s easy to understand why reproductive traits would be subject to strong selection because of their direct effects on fitness. II. As we might expect, there are three basic types of mammalian reproduction. These correspond to the three major groups of mammals. Differences among these types reflect the degree of intimacy of fetal-maternal contact. A. Monotreme Type - Retain the primitive amniote pattern, but add a period of lactation. - Egg is very large at ovulation, about 3 mm. It moves down the oviduct where it is fertilized, then it’s coated with albumin, then a shell is laid down by a shell gland, and the shell is mineralized (with CaCO3). - Embryo spends 2-3 weeks in the uterus, then it’s laid. It’s large, 15 mm, when it’s laid. - Embryo growth is supported entirely by the yolk that’s enclosed in the egg. B. Metatherian type: - In some superficial ways it’s similar to monotremes, in that metatherians retain a shell membrane around the fertilized egg; a shell gland is present in Müllerian ducts. - However the shell is never mineralized and young are born live after only ca. 12 days, as we’ve seen, they are very altricial (poorly developed). - Initial intrauterine growth is supported by maternally by a placenta. - Metatherian placenta is the choriovitelline type - yolk-sac placenta (Eutherian placenta is called chorioallantoic placenta) C. Placenta Formation - Let’s look at formation of the placenta in Meta- & Eutherians. -

Equine Placenta – Marvelous Organ and a Lethal Weapon
Equine placenta – marvelous organ and a lethal weapon Malgorzata Pozor, DVM, PhD, Diplomate ACT Introduction Placenta has been defined as: „an apposition between parent (usually maternal) and fetal tissue in order to establish physiological exchange” (1). Another definition of this important organ was proposed by Steven and Morris: „a device consisting of one or more transport epithelia located between fetal and maternal blood supply” (2). The main function of placenta is to provide an interface between the dam and the the fetus and to allow the metabolic exchange of the the nutrients, oxygen and waste material. The maternal circulation is brought into a close apposition to the fetal circulation, while a separation of these two circulatory systems remain separated (3). A degree and complexity of this „intimate relationship” varies greately between species mostly due to the structural diversity of the extraembryonic membranes of the vertebrates. The early feto-maternal exchange in the equine pregnancy is established as early as on day 22 after fertilization. The fetal and choriovitellin circulations are already present, the capsule ruptures and the allantois is already visible (4). The allantois starts expanding by day 32 and vascularizes approximately 90% of the chorion and fuses with it to form chorioallantois by day 38 of gestation (5). The equine placenta continues increasing its complexity till approximately day 150 of gestation. Equids have epitheliochorial placenta, there are six leyers separating maternal and fetal circulation, and there are no erosion of the luminal, maternal epithelium, like in ruminants (6). Thousands of small chorionic microvilli develop and penetrate into endometrial invaginations. -

From Trophoblast to Human Placenta
From Trophoblast to Human Placenta (from The Encyclopedia of Reproduction) Harvey J. Kliman, M.D., Ph.D. Yale University School of Medicine I. Introduction II. Formation of the placenta III. Structure and function of the placenta IV. Complications of pregnancy related to trophoblasts and the placenta Glossary amnion the inner layer of the external membranes in direct contact with the amnionic fluid. chorion the outer layer of the external membranes composed of trophoblasts and extracellular matrix in direct contact with the uterus. chorionic plate the connective tissue that separates the amnionic fluid from the maternal blood on the fetal surface of the placenta. chorionic villous the final ramification of the fetal circulation within the placenta. cytotrophoblast a mononuclear cell which is the precursor cell of all other trophoblasts. decidua the transformed endometrium of pregnancy intervillous space the space in between the chorionic villi where the maternal blood circulates within the placenta invasive trophoblast the population of trophoblasts that leave the placenta, infiltrates the endo– and myometrium and penetrates the maternal spiral arteries, transforming them into low capacitance blood channels. Sunday, October 29, 2006 Page 1 of 19 From Trophoblasts to Human Placenta Harvey Kliman junctional trophoblast the specialized trophoblast that keep the placenta and external membranes attached to the uterus. spiral arteries the maternal arteries that travel through the myo– and endometrium which deliver blood to the placenta. syncytiotrophoblast the multinucleated trophoblast that forms the outer layer of the chorionic villi responsible for nutrient exchange and hormone production. I. Introduction The precursor cells of the human placenta—the trophoblasts—first appear four days after fertilization as the outer layer of cells of the blastocyst. -
Placenta-4-2-15.Pdf
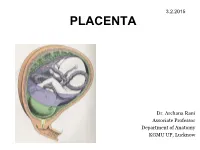
3.2.2015 PLACENTA Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow Implantation • Begins on the 6th day after fertilization • Embedding of blastocyst in the wall of uterus • Disappearance of zona pellucida • Cells of the trophoblast stick to the endometrium • After implantation of the embryo, the uterine endometrium is called the decidua Implantation Site of implantation • Normal site - At the junction of fundus and posterior wall of uterus - At upper uterine segment Interstitial Implantation The Decidua After implantation of the embryo, the uterine endometrium is called the decidua. Subdivisions of Decidua Formation of Chorionic Villi • Villi are the essential functional elements of the placenta. • Small finger like processes. • Surrounded by maternal blood. • Fetal blood circulates in their substance through capillaries. • Called as Chorionic villi as arises as offshoots from chorion. Stages in formation of Chorionic villi Early stages in formation of Chorionic villi (9th day) Formation of Chorionic villi Lacunar spaces filled with maternal blood Primary Villus (Day 13-14) All elements (syncytium, cytotrophoblast and extraembryonic mesoderm) take part in formation of chorionic villi. Three stages in formation of chorionic villi are seen: Primary villus: central core of cytotrophoblast covered by a layer of syncytiotrophoblast. Adjoining villi are separated by an intervillous space. Secondary Villus (Early 3rd week) Central core of extraembryonic mesoderm covered successively by cyto and syncytiotrophoblasts. Tertiary Villus (End of 3rd week) Appearance of blood vessels in the mesoderm forming core of each villus. Formation of cytotrophoblastic shell Anchoring villi & its subdivisions Fully formed Placenta (4th month) DEFINITION • Placenta is a fetomaternal organ which is the primary site of nutrient and gas exchange between the fetus and the mother. -

Placental Pathology: Its Impact on Explaining Prenatal and Perinatal Death
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2004 Placental pathology: its impact on explaining prenatal and perinatal death Stallmach, Thomas ; Hebisch, Gundula Abstract: This review considers six main situations in which pathologists are expected to report and interpret placental messages for obstetricians, neonatologists and, indirectly, parents: (1) abortion is the body’s corrective response to the embryonic defect suggested by malformed chorionic villi; (2) in- fection causing chorionic villous inflammation is specific and haematogenous; pathogen identification is mandatory, in contrast to chorioamnionitis caused by increased local immunosuppression allowing indis- criminate bacterial entry; (3) prematurity and (4) intrauterine growth restriction are often associated with pregnancy-specific disease (pre-eclampsia) or pre-existing maternal conditions (systemic lupus); parental studies may improve outcome in subsequent pregnancies; (5) intrauterine death near term is often due to placental dysmaturity featuring a severely reduced number of syncytiocapillary membranes; it accounts for the death in utero of 3 in 1000 pregnancies; detection helps to minimise recurrence in subsequent pregnancies; (6) twins are best confirmed as monozygous by the absence of chorionic tissue in thedivid- ing membranes; most monochorionic twins have vascular connections whose detailed analysis is requested only if there are inter-twin differences in growth and colour. From a formal point of view, many morebits of pathology than discussed in this review can be found in placentas and, with the advances in ultrasonog- raphy, might even be seen prior to birth. The extent of such a disturbance might ultimately affect fetal growth, which is amenable to prenatal detection offering the chances for an appropriate management. -

LORENNA CARDOSO REZENDE Biologia Da Reprodução Em Tatus: Análise Morfológica Do Aparelho Reprodutor Feminino Da Espécie
LORENNA CARDOSO REZENDE Biologia da Reprodução em Tatus: Análise Morfológica do Aparelho Reprodutor Feminino da Espécie Euphractus sexcinctus e Análise Morfológica Placentária Comparativa entre as Espécies Chaetophractus villosus, Chaetophractus vellerosus e Euphractus sexcinctus SÃO PAULO 2011 LORENNA CARDOSO REZENDE Biologia da Reprodução em Tatus: Análise Morfológica do Aparelho Reprodutor Feminino da Espécie Euphractus sexcinctus e Análise Morfológica Placentária Comparativa entre as Espécies Chaetophractus villosus, Chaetophractus vellerosus e Euphractus sexcinctus Tese apresentada ao Programa de Pós- graduação em Anatomia dos Animais Domésticos e Silvestres da Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo para obtenção do título de Doutor em Ciências Departamento: Cirurgia Área de concentração: Anatomia dos Animais Domésticos e Silvestres Orientadora: Profa. Dra. Maria Angélica Miglino SÃO PAULO 2011 Autorizo a reprodução parcial ou total desta obra, para fins acadêmicos, desde que citada a fonte. DADOS INTERNACIONAIS DE CATALOGAÇÃO‐NA‐PUBLICAÇÃO (Biblioteca Virginie Buff D’Ápice da Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo) T.2504 Rezende, Lorenna Cardoso FMVZ Biologia da reprodução em tatus: análise morfológica do aparelho reprodutor feminino da espécie Euphractus sexcinctus e análise morfológica placentária comparativa entre as espécies Chaetophractus villosus, Chaetophractus vellerosus e Euphractus sexcinctus / Lorenna Cardoso Rezende.‐‐ 2011. 141 f. : il. Tese (Doutorado) -

Chapter 29 *Lecture Powerpoint
Chapter 29 *Lecture PowerPoint Human Development and Aging *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Miraculous aspect of human life is the transformation of a one-celled fertilized egg into an independent, fully developed individual • Embryology—the study of prenatal development • Developmental biology—a broader science that embraces changes in form and function from fertilized egg through old age 29-2 Fertilization and the Preembryonic Stage • Expected Learning Outcomes – Describe the process of sperm migration and fertilization. – Explain how an egg prevents fertilization by more than one sperm. – Describe the major events that transform a fertilized egg into an embryo. – Describe the implantation of the preembryo in the uterine wall. 29-3 Fertilization and the Preembryonic Stage • Embryo—term has different meanings – Stages beginning with the fertilized egg or the two-cell stage produced by its first division – Individual 16 days old when it consists of the three primary germ layers • Ectoderm, mesoderm, and ectoderm • Embryogenesis—events leading up to this stage • Preembryonic stage is the first 16 days after fertilization 29-4 Sperm Migration • Egg must be fertilized within 12 to 24 hours of ovulation, if it is to survive • Sperm must encounter the egg somewhere in the distal one-third of the uterine tube • Vast majority of sperm do not make it to egg – Destroyed -

Review Article Placenta Accreta Spectrum: a Review of Pathology, Molecular Biology, and Biomarkers
Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Item Type Article Authors Bartels, Helena C;Postle, James D;Downey, Paul;Brennan, Donal J Citation Helena C. Bartels, James D. Postle, Paul Downey, and Donal J. Brennan, “Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers,” Disease Markers, vol. 2018, Article ID 1507674, 11 pages, 2018. https:// doi.org/10.1155/2018/1507674. DOI 10.1155/2018/1507674 Publisher Hindawi Journal Disease Markers Rights Attribution-NonCommercial-NoDerivs 3.0 United States Download date 07/10/2021 06:20:50 Item License http://creativecommons.org/licenses/by-nc-nd/3.0/us/ Link to Item http://hdl.handle.net/10147/623961 Find this and similar works at - http://www.lenus.ie/hse Hindawi Disease Markers Volume 2018, Article ID 1507674, 11 pages https://doi.org/10.1155/2018/1507674 Review Article Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers 1 1 1 1,2 Helena C. Bartels , James D. Postle, Paul Downey, and Donal J. Brennan 1National Maternity Hospital, Holles Street, Dublin 2, Ireland 2UCD School of Medicine, National Maternity Hospital, Holles Street, Dublin 2, Ireland Correspondence should be addressed to Donal J. Brennan; [email protected] Received 20 March 2018; Accepted 10 June 2018; Published 3 July 2018 Academic Editor: Irene Rebelo Copyright © 2018 Helena C. Bartels et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Placenta accreta spectrum (PAS) is a condition of abnormal placental invasion encompassing placenta accreta, increta, and percreta and is a major cause of severe maternal morbidity and mortality. -

Quantitative Morphometric Study of the Chorionic Villi in Hypertensive
The Egyptian Journal of Hospital Medicine (October 2017) Vol. 69 (4), Page 2315-2322 Quantitative Morphometric Study of The Chorionic Villi in Hypertensive Mothers Abdulrhman Saleh Dairi1, Wagih Gamal Elbarrany2, Amna Abdul Rahim Moulana3, Ahmad Sami A Himayda1, Iyad M.Hakeem1 1Medical Intern, Faculty of Medicine and Surgery, Umm Al-Qura University, 2Professor of Anatomy, Faculty of Medicine and Surgery, Umm Al-Qura University, 3Consultant of Anatomical Pathology, Maternity and Children’s Hospital, Ministry of Health, Makkah, Saudi Arabia. Corresponding Author: Abdulrhman Saleh Dairi, email:[email protected], mobile:+966558306306 ABSTRACT Background: Several studies have reported that mothers with pregnancy induced hypertension or those suffering from hypertensive disorders have abnormalities in histological features of the placenta. The fetus connection with the mother is through chorionic villi. Besides several other histological features changes in the chorionic villi have also been reported. This lead to the reduced supply of the necessary nutritional elements for the fetus. The aim of the Study: The principal objective of this study was to evaluate morphometric changes in the placenta of expecting mothers have hypertensive disorders of pregnancy and women without any symptoms of hypertension. As the placenta is capillary-rich region and any physiological change can adversely affect the fetal health. Patients and Methods: In this study, a total of 84 expecting mothers were recruited. Among these 42 have hypertensive symptoms before pregnancy whereas the other 42 have their blood pressure in normal ranges. Among the 42 hypertensive women, only 13 met the study inclusion criteria,i.e., blood pressure in the range of 140/90 mmHg in the 30th week of the pregnancy. -
![The Process of Implantation of Embryos in Primates [1]](https://docslib.b-cdn.net/cover/3316/the-process-of-implantation-of-embryos-in-primates-1-3923316.webp)
The Process of Implantation of Embryos in Primates [1]
Published on The Embryo Project Encyclopedia (https://embryo.asu.edu) The Process of Implantation of Embryos in Primates [1] By: Wolter, Justin M. Keywords: Reproduction [2] Human development [3] Fertilization [4] Implantation is a process in which a developing embryo, moving as a blastocyst [5] through a uterus [6], makes contact with the uterine wall and remains attached to it until birth. The lining of the uterus [6] (endometrium [7]) prepares for the developing blastocyst [5] to attach to it via many internal changes. Without these changes implantation [8] will not occur, and the embryo sloughs off during menstruation [9]. Such implantation [8] is unique to mammals, but not all mammals exhibit it. Furthermore, of those mammals that exhibit implantation [8], the process differs in many respects between those mammals in which the females have estrous cycles, and those mammals in which the females have menstrual cycles. Females in the different species of primates, including humans [10], have menstrual cycles, and thus similar processes of implantation [8]. Before embryogenesis [11] begins, the ovary [12] releases an unfertilized egg [13] cell, called an oocyte [14], which then travels down the fallopian tube. The egg [13] is enveloped in an extracellular matrix called the zona pellucida [15]. Sperm can fertilize the egg [13] in the zona pellucida [15] (ZP), which prevents the fertilized egg [16], called a zygote [17], from adhering to the wall of the fallopian tube. If the zygote [17] implants in any area besides the uterus [6], the result is an ectopic pregnancy [18]. This condition prevents the complete development of the embryo, and it can cause fatal hemorrhaging in the preganant female. -

17. Formation and Role of Placenta
17. FORMATION AND ROLE OF PLACENTA Joan W. Witkin, PhD Dept. Anatomy & Cell Biology, P&S 12-432 Tel: 305-1613 e-mail: [email protected] READING: Larsen, 3rd ed. pp. 20-22, 37-44 (fig. 2-7, p. 45), pp. 481-490 SUMMARY: As the developing blastocyst hatches from the zona pellucida (day 5-6 post fertilization) it has increasing nutritional needs. These are met by the development of an association with the uterine wall into which it implants. A series of synchronized morphological and biochemical changes occur in the embryo and the endometrium. The final product of this is the placenta, a temporary organ that affords physiological exchange, but no direct connection between the maternal circulation and that of the embryo. Initially cells in the outer layer of the blastocyst, the trophoblast, differentiate producing an overlying syncytial layer that adheres to the endometrium. The embryo then commences its interstitial implantation as cells of the syncytiotrophoblast pass between the endometrial epithelial cells and penetrate the decidualized endometrium. The invading embryo is first nourished by secretions of the endometrial glands. Subsequently the enlarging syncytiotrophoblast develops spaces that anastomose with maternal vascular sinusoids, forming the first (lacunar) uteroplacental circulation. The villous placental circulation then develops as fingers of cytotrophoblast with its overlying syncytiotrophoblast (primary villi) extend from the chorion into the maternal blood space. The primary villi become secondary villi as they are invaded by extraembryonic mesoderm and finally tertiary villi as embryonic blood vessels develop within them. During the first trimester of pregnancy cytotrophoblasts partially occlude the uterine vessels such that only plasma circulates in the intervillous space. -

The Placenta Learning Module
The placenta Learning module Developed by Carolyn Hammer Edited by Fabien Giroux Diagrams by Dr Yockell –Lelievre where indicated The placenta – Learning module Table of content 1) Introduction…………………………………………………………………………...…3 2) Anatomy and Physiology…………………………………………………….………...6 3) Roles and Functions…………………………………………………………..………23 4) Development and formation…………………………………………………………..35 5) What happens after birth…………………………………………………………...…44 6) What happens when things go wrong……………………………………………....46 7) Interesting facts about pregnancy…………………………………..……………….57 8) Testing what you know………………………………………..……………………...62 2 The placenta – Learning module Introduction 3 The placenta – Learning module What is the placenta? •The placenta is a: “vascular (supplied with blood vessels) organ in most mammals that unites the fetus to the uterus of the mother. It mediates the metabolic exchanges of the developing individual through an intimate association of embryonic tissues and of certain uterine tissues, serving the functions of nutrition, respiration, and excretion.” (Online Britannica Encyclopaedia) •The placenta is also known as a hemochorical villous organ meaning that the maternal blood comes in contact with the chorion and that villi protrude out of this same structure. As the fetus is growing and developing, it requires a certain amount of gases and nutrients to help support its needs throughout pregnancy. Because the fetus is unable to do so on its own, it is the placenta that carries out this function. http://health.allrefer.com/health/plac enta-abruptio-placenta.html 4 The placenta – Learning module What are the main roles of the placenta? •The placenta provides the connection between fetus and mother in order to help carry out many different functions that it is incapable to do alone.