Mussels As a Model System for Integrative Ecomechanics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019-The Benthic Sea-Silk-Thread Displacement of a Sessile Bivalve
The benthic sea-silk-thread displacement of a sessile bivalve, Pinctada ANGOR UNIVERSITY imbricata radiata (Leach, 1819) in the Arabian-Persian Gulf Giraldes, Bruno Welter; Leitao, Alexander; Smyth, David PLoS ONE DOI: 10.1371/journal.pone.0215865 PRIFYSGOL BANGOR / B Published: 01/05/2019 Publisher's PDF, also known as Version of record Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Giraldes, B. W., Leitao, A., & Smyth, D. (2019). The benthic sea-silk-thread displacement of a sessile bivalve, Pinctada imbricata radiata (Leach, 1819) in the Arabian-Persian Gulf. PLoS ONE, 14(5), [e0215865]. https://doi.org/10.1371/journal.pone.0215865 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 28. Sep. 2021 RESEARCH ARTICLE -

Fall ’17 Footwear Trends Smart Textiles Tech Advances Trade Show Previews & Recaps Making It in America
TEXTILE INSIGHT Trends In Apparel & Footwear Design and Innovation • January/February 2017 iNvEnToLoGy HOW THE TEXTILE INDUSTRY IS INVENTING ITS FUTURE Fall ’17 Footwear Trends Smart Textiles Tech Advances Trade Show Previews & Recaps Making it in America A FORMULA4 MEDIA PUBLICATION TEXTILEINSIGHT.COM A breakthrough fiber innovation you have to feel to believe. Eastman Avra performance fibers wick better, dry faster, and keep you at your coolest and most comfortable. AVRAfromEastman.com ©2017 Eastman Chemical Company. Eastman brands referenced herein are trademarks of Eastman Chemical Company or one of its subsidiaries. The ® used on Eastman brands denotes registered trademark status in the U.S.; marks may also be registered internationally. JANUARY/FEBRUARY 2017 JANUARY/FEBRUARY Executive Editor Mark Sullivan [email protected] 646-319-7878 Editor /Associate Publisher Emily Walzer [email protected] Managing Editor Cara Griffin Art Director Francis Klaess Associate Art Director Mary McGann Contributing Editors Suzanne Blecher Kurt Gray Jennifer Ernst Beaudry Kathlyn Swantko Publisher Jeff Nott [email protected] 516-305-4711 Advertising Jeff Gruenhut [email protected] 404-467-9980 Christina Henderson 516-305-4710 [email protected] Troy Leonard [email protected] 352-624-1561 Katie O’Donohue [email protected] 828-244-3043 Sam Selvaggio [email protected] 212-398-5021 Production Brandon Christie 516 305-4710 [email protected] Business Manager Marianna Rukhvarger 516-305-4709 [email protected] Biosteel is the latest knit upper shoe technology from Adidas set to debut this year. Subscriptions store.formula4media.com Formula4 Media Publications 6 / In the Market 40 / Made in America Sports Insight The New Year begins with trade show previews, reports on Before “Think locally, act globally” became a popular buzz- Outdoor Insight Footwear Insight Performance Days, advances in digital textiles printing, and phrase, IDEAL Fastener was putting the strategy to work. -

Sea-Silk Based Nanofibers and Their Diameter Prediction THERMAL SCIENCE: Year 2019, Vol
Tian, D., et al.: Sea-Silk Based Nanofibers and Their Diameter Prediction THERMAL SCIENCE: Year 2019, Vol. 23, No. 4, pp. 2253-2256 2253 SEA-SILK BASED NANOFIBERS AND THEIR DIAMETER PREDICTION by * Dan TIAN, Chan-Juan ZHOU, and Ji-Huan HE National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou, China Original scientific paper https://doi.org/10.2298/TSCI1904253T Diameter of sea-silk based nanofibers prepared by electrospinning is closely re- lated to the concentrations of sea-silk solution. A mathematical model is estab- lished according the mass conservation law in fluid mechanics to predict the di- ameter of fibers, and the MATLAB software is used to fit the experiment value. The results show that the fitted equation is quite accurate and efficient for estimating the diameter of fibers with different concentrations. Key words: electrospinning, sea-silk, mathematical model, nanofiber, fiber’s diameter. Introduction Electrospinning is an effective way to prepare nanofibers, it is a fabrication process that uses an electric field to control the deposition of polymer fibers onto a receptor [1-8]. In electrospinning process, the diameter of nanofiber is determined by many factors, like volt- age, viscosity of solution, receptor’s distance, environment temperature, environment humidi- ty, etc. [9, 10]. Nanofiber’s diameter and morphology can also be controlled by additives [11]. The sea silk is one of the oldest natural silks, which has a history of more than 5000 years [12-16], and now we can produce artificial sea silk through Mytilus edulis. To this end, we extract protein from Mytilus edulis and then use it as an additive for electrospinning, this maybe has some effects on the morphology of fibers. -

Natural, Artificial and Synthetic Fibres: Because There Are Differences...There Are Many...And It Is Important to Know Them
Deepening T.SILK | Natural, artificial and synthetic fibers. Because there are the differences… there are many… and it is important to know them Natural, artificial and synthetic fibres: because there are differences...there are many...and it is important to know them. Natural fibres are those obtained through simple mechanical processes that do not modify the structure. They differ between natural fibres of animal origin (wool, angora fur, camel fur, cashmere fur, mohair fur, alpaca fur, llama fur, vicuna fur, bison fur, qiviut fur, sea silk, down) and vegetable (cotton, linen, hemp, jute, ramie or nettle, sisal, coconut, broom, hibiscus, manila, straw, bamboo, soy, kapok). - Silk Chemically: • Fibroin – highly biocompatible and biodegradable natural polymer protein (carbon 47.6% Hydrogen 6.39% Nitrogen 18.33% Oxygen 27.68%) • Sericin – natural protein with a unique affinity to other human proteins (carbon 46.50% Hydrogen 6.04% Nitrogen 16.50% Oxygen 30.96%) Amino acid components: GLYCINE (Helps to trigger the oxygen release process) - ALANINE (Important source of energy for muscle tissue) - SERINE (Source of glucose storage in the liver and muscles) - ASPARTIC ACID (Helps the expulsion of harmful ammonia from the body) - GLUTAMIC ACID (Considered as a natural "food for the mind") - VALINE (Stimulates mental vigour and muscle coordination) - PROLINE (Important for the correct functioning of joints and tendons) - THREONINE (Important constituent of collagen) - LYSINE (Ensures adequate calcium absorption) - ARGININE (Improves the immune -

The Peculiar Protein Ultrastructure of Fan Shell and Pearl Oyster Byssus
Soft Matter View Article Online PAPER View Journal | View Issue A new twist on sea silk: the peculiar protein ultrastructure of fan shell and pearl oyster byssus† Cite this: Soft Matter, 2018, 14,5654 a a b b Delphine Pasche, * Nils Horbelt, Fre´de´ric Marin, Se´bastien Motreuil, a c d Elena Macı´as-Sa´nchez, Giuseppe Falini, Dong Soo Hwang, Peter Fratzl *a and Matthew James Harrington *ae Numerous mussel species produce byssal threads – tough proteinaceous fibers, which anchor mussels in aquatic habitats. Byssal threads from Mytilus species, which are comprised of modified collagen proteins – have become a veritable archetype for bio-inspired polymers due to their self-healing properties. However, threads from different species are comparatively much less understood. In particular, the byssus of Pinna nobilis comprises thousands of fine fibers utilized by humans for millennia to fashion lightweight golden fabrics known as sea silk. P. nobilis is very different from Mytilus from an ecological, morphological and evolutionary point of view and it stands to reason that the structure– Creative Commons Attribution 3.0 Unported Licence. function relationships of its byssus are distinct. Here, we performed compositional analysis, X-ray diffraction (XRD) and transmission electron microscopy (TEM) to investigate byssal threads of P. nobilis, as well as a closely related bivalve species (Atrina pectinata) and a distantly related one (Pinctada fucata). Received 20th April 2018, This comparative investigation revealed that all three threads share a similar molecular superstructure Accepted 18th June 2018 comprised of globular proteins organized helically into nanofibrils, which is completely distinct from DOI: 10.1039/c8sm00821c the Mytilus thread ultrastructure, and more akin to the supramolecular organization of bacterial pili and F-actin. -

ATR 61 Cover Yellow Red.Jpg
Current Research in Textile Archaeology along the Nile Nosch, Marie Louise Bech Published in: Archaeological Textiles Review Publication date: 2019 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Nosch, M. L. B. (2019). Current Research in Textile Archaeology along the Nile. Archaeological Textiles Review, 61, 26-28. Download date: 09. Apr. 2020 Contents Archaeological Textiles Review Editorial 2 ATR is published by the Society Friends of ATN, hosted by Centre for Textile Articles Research in Copenhagen. Editors: Spinning for the gods? Preliminary 3 Eva Andersson Strand observations on prehistoric textile production Karina Grömer at Hierakonpolis, Egypt Jane Malcolm-Davies Anne Drewsen Ulla Mannering Textiles from Zawaydah, Naqada, Upper Egypt 14 Margarita Gleba, Mathieu Boudin Scientifi c committ ee: and Grazia A. Di Pietro John Peter Wild, UK Lise Bender Jørgensen, Norway Late Antique textiles from Egypt in the 24 Elisabeth Wincott Heckett , Ireland Ny Carlsberg Glyptotek, Copenhagen Johanna Banck-Burgess, Germany Cecilie Brøns, Ina Vanden Berghe and Irene Skals Tereza Štolcová, Slovakia Heidi Sherman, USA Blue dyed textiles in Early Iron Age Europe: 42 Claudia Merthen, Germany Accessible or exclusive? Christina Margariti, Greece Patricia Hopewell and Susanna Harris Layout: Karina Grömer The Textiles of Üzüür Gyalan: Towards the 56 Cover: Charlott e Rimstad identifi cation of a nomadic weaving tradition in (Image: NCG Collection ÆIN 956, the Mongolian Altai Copenhagen – Late Antique textile) Kristen Rye Pearson, Chuluunbat Mönkhbayar, Galbadrakh Enkhbat and Jamsranjav Bayarsaikhan Print: Grafi sk University of Copenhagen Time looms over us: Observations from an 71 experimental comparison of medieval English loom-types Subscription information: To purchase Gwendoline Pepper a copy of the latest Archaeological Textiles Review, please visit: Nets – Knots – Lace: Early 16th century www.webshophum-en.ku.dk/shop/ 88 archaeological-textiles-333c1.html. -

To Be Published in Gazette of India, Extra Ordinary, Part 1, Section1
To be published in Gazette of India, Extra ordinary, Part 1, Section1 F. No. 15/16/2013-DGAD Government of India Ministry of Commerce & Industry Department of Commerce (Directorate General of Anti Dumping & Allied Duties) 4th Floor, Jeevan Tara Building, 5, Parliament Street, New Delhi 110001 Dated the 23rd March, 2015 NOTIFICATION (Final Findings) Subject: Final Findings in the sunset review of anti-dumping duty imposed on the imports of Acrylic Fibre originating in or exported from Korea RP and Thailand-reg. A. BACKGROUND OF THE CASE 1. No. 15/16/2013-DGAD:- Whereas having regard to the Customs Tariff Act, 1975 as amended from time to time (herein after referred to as the Act) and the Customs Tariff (Identification, Assessment and Collection of Duty on Dumped Articles and for Determination of Injury) Rules, 1995 (herein after referred to as the AD Rules), the Designated Authority (herein after referred to as the Authority), initiated the original anti dumping investigation in respect of the imports of Acrylic Fibre (hereinafter referred to as the subject goods) originating in or exported from USA, Thailand and Korea RP on 13.9.1996 and definitive anti dumping duty was recommended vide Final Findings Notification No. 47/ADD/1W dated 14.10.1997. The Central Government had imposed the anti dumping duty. The sunset review of the anti dumping duty so imposed against USA, Thailand and Korea RP was initiated by the Authority vide Notification No. 26/1/2001-DGAD dated 07.08.2001 and the Final Findings were issued vide Notification No. 26/1/2001-DGAD dated 06.08.2002. -

U.S. EPA, Pesticide Product Label, CUTTER INSECT REPELLENT
U.S. ENVIRONMENTAL PROTECTION AGENCY EPA Reg. Date of Issuance: Office of Pesticide Programs Number: Registration Division (H750SC) 401 "M" St., S.W. 121-91 Washington, D.C. 20460 OCT 2 a 2005 Term of Issuance: NOTICE OF PESTICIDE: Conditional _x__ Registration Reregistration Name of Pesticide Product: (under FIFRA. as amended) CUTTER Insect Repellent 15KP Name and Address of Registrant (include ZIP Code): Spectrum Division of United Industries P.O. Box 142642 St. Louis, MO 63114-0642 Note: Changes in labeling differing in substance from that accepted in connection with this registration must be submitted to and accepted by the Registration Division prior to use of the label in commerce. In any correspondence on this product always refer to the above EPA registration number. On the basis of information furnished by the registrant, the above named pesticide is hereby registered/reregistered under the Federal Insecticide, Fungicide and Rodenticide Act. Registration is in no way to be construed as an endorsement or recommendation of this product by the Agency. In order to protect health and the environment, the Administrator, on his motion, may at any time suspend or cancel the registration of a pesticide in accordance with the Act. The acceptance of any name in connection with the registration of a product under this Act is not to be construed as giving the registrant a right to exclusive use of the name or to its use if it has been covered by others. This product is conditionally registered in accordance with FIFRA sec. 3(c) (7) (A) provided that you: 1. -
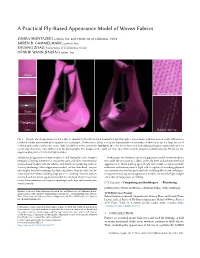
A Practical Ply-Based Appearance Model of Woven Fabrics
A Practical Ply-Based Appearance Model of Woven Fabrics ZAHRA MONTAZERI, Luxion, Inc. and University of California, Irvine SØREN B. GAMMELMARK, Luxion, Inc. SHUANG ZHAO, University of California, Irvine HENRIK WANN JENSEN, Luxion, Inc. Photo Ours Frontlit close-up Photo Ours Backlit close-up Fig. 1. Our ply-based appearance model is able to simulate both reflected and transmitted light through complex weave patterns as seen on the left andour rendered results match with photographs of a real sample. Furthermore, it has a compact representation that makes it efficient to use for large pieces of clothing such as the scarf shown on the right. In addition to the anisotropic highlights, the color due to front and back lighting changes considerably and our model reproduces the color shift seen in the photographs. The images on the right are close-ups of the scarf showing the individual yarns. Pleaseseethe supplementary video for the full light rotation. Simulating the appearance of woven fabrics is challenging due to the complex In this paper, we introduce a practical appearance model for woven fabrics. interplay of lighting between the constituent yarns and fibers. Conventional We model the structure of a fabric at the ply level and simulate the local surface-based models lack the fidelity and details for producing realistic appearance of fibers making up each ply. Our model accounts for both close-up renderings. Micro-appearance models, on the other hand, can pro- reflection and transmission of light and is capable of matching physical duce highly detailed renderings by depicting fabrics fiber-by-fiber, but be- measurements better than prior methods including fiber based techniques. -

Textile Design: a Suggested Program Guide
DOCUMENT RESUME CI 003 141 ED 102 409 95 Program Guide.Fashion TITLE Textile Design: A Suggested Industry Series No. 3. Fashion Inst. of Tech.,New York, N.T. INSTITUTION Education SPONS AGENCY Bureau of Adult,Vocational, and Technictl (DREW /OE), Washington,D.C. PUB DATE 73 in Fashion Industry NOTE 121p.; For other documents Series, see CB 003139-142 and CB 003 621 Printing AVAILABLE FROM Superintendent of Documents,U.S. Government Office, Washington, D.C.20402 EDRS PRICE NP -$0.76 HC-$5.70 PLUS POSTAGE Behavioral Objectives; DESCRIPTORS Adult, Vocational Education; Career Ladders; *CurriculumGuides; *Design; Design Crafts; EducationalEquipment; Employment Opportunities; InstructionalMaterials; *Job Training; Needle Trades;*Occupational Rome Economics; OccupationalInformation; Program Development; ResourceGuides; Resource Units; Secondary Education;Skill Development;*Textiles Instruction IDENTIFIERS *Fashion Industry ABSTRACT The textile designguide is the third of aseries of resource guidesencompassing the various five interrelated program guide is disensions of the fashionindustry. The job-preparatory conceived to provide youthand adults withintensive preparation for and also with careeradvancement initial entry esploysent jobs within the textile opportunities withinspecific categories of provides an overviewof the textiledesign field, industry. The guide required of workers. It occupational opportunities,and cospetencies contains outlines of areasof instruction whichinclude objectives to suggestions for learning be achieved,teaching -

Cyromazine) During Woolscouring and Its Effects on the Aquatic Environment the Fate of Vetrazin® (Cyromazine) During
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. THE FATE OF VETRAZIN@ (CYROMAZINE) DURING WOOLSCOURING AND ITS EFFECTS ON THE AQUATIC ENVIRONMENT THE FATE OF VETRAZIN® (CYROMAZINE) DURING WOOLSCOURING AND ITS EFFECTS ON THE AQUATIC ENVIRONMENT A thesis submitted in fulfilment of the requirements for the degree of DOCTOR OF PHILOSOPHY in AQUATIC TOXICOLOGY at LINCOLN UNIVERSITY P.W. Robinson 1995 " ; " i Abstract of a thesis submitted in partial fulfllment of the requirement for the Degree of Doctor of Philosophy THE FATE OF VETRAZIN® (CYROMAZINE) DURING WOOLSCOURING AND ITS EFFECTS ON THE AQUATIC ENVIRONMENT by P.W. Robinson A number of ectoparasiticides are used on sheep to protect the animals from ill health associated with infestations of lice and the effects of fly-strike. Most of the compounds currently in use are organophosphate- or pyrethroid-based and have been used for 15-20 years, or more. In more recent times, as with other pest control strategies, there has been a tendency to introduce 'newer' pesticides, principally in the form of insect growth regulators (IGRs). -

Natural, Artificial and Synthetic Fibres: Because There Are Differences...There Are Many...And It Is Important to Know Them
Approfondimento T.Silk | Fibre naturali, artificiali e sintetiche. Perché le differenze ci sono…sono tante…ed è importante conoscerle Natural, artificial and synthetic fibres: because there are differences...there are many...and it is important to know them. Natural fibres are those obtained through simple mechanical processes that do not modify the structure. They differ between natural fibres of animal origin (wool, angora fur, camel fur, cashmere fur, mohair fur, alpaca fur, llama fur, vicuna fur, bison fur, qiviut fur, sea silk, down) and vegetable (cotton, linen, hemp, jute, ramie or nettle, sisal, coconut, broom, hibiscus, manila, straw, bamboo, soy, kapok). - Silk Chemically: • Fibroin – highly biocompatible and biodegradable natural polymer protein (carbon 47.6% Hydrogen 6.39% Nitrogen 18.33% Oxygen 27.68%) • Sericin – natural protein with a unique affinity to other human proteins (carbon 46.50% Hydrogen 6.04% Nitrogen 16.50% Oxygen 30.96%) Amino acid components: GLYCINE (Helps to trigger the oxygen release process) - ALANINE (Important source of energy for muscle tissue) - SERINE (Source of glucose storage in the liver and muscles) - ASPARTIC ACID (Helps the expulsion of harmful ammonia from the body) - GLUTAMIC ACID (Considered as a natural "food for the mind") - VALINE (Stimulates mental vigour and muscle coordination) - PROLINE (Important for the correct functioning of joints and tendons) - THREONINE (Important constituent of collagen) - LYSINE (Ensures adequate calcium absorption) - ARGININE (Improves the immune response to