The EWS/ATF1 Fusion Protein Contains a Dispersed Activation Domain That Functions Directly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

A Flexible Microfluidic System for Single-Cell Transcriptome Profiling
www.nature.com/scientificreports OPEN A fexible microfuidic system for single‑cell transcriptome profling elucidates phased transcriptional regulators of cell cycle Karen Davey1,7, Daniel Wong2,7, Filip Konopacki2, Eugene Kwa1, Tony Ly3, Heike Fiegler2 & Christopher R. Sibley 1,4,5,6* Single cell transcriptome profling has emerged as a breakthrough technology for the high‑resolution understanding of complex cellular systems. Here we report a fexible, cost‑efective and user‑ friendly droplet‑based microfuidics system, called the Nadia Instrument, that can allow 3′ mRNA capture of ~ 50,000 single cells or individual nuclei in a single run. The precise pressure‑based system demonstrates highly reproducible droplet size, low doublet rates and high mRNA capture efciencies that compare favorably in the feld. Moreover, when combined with the Nadia Innovate, the system can be transformed into an adaptable setup that enables use of diferent bufers and barcoded bead confgurations to facilitate diverse applications. Finally, by 3′ mRNA profling asynchronous human and mouse cells at diferent phases of the cell cycle, we demonstrate the system’s ability to readily distinguish distinct cell populations and infer underlying transcriptional regulatory networks. Notably this provided supportive evidence for multiple transcription factors that had little or no known link to the cell cycle (e.g. DRAP1, ZKSCAN1 and CEBPZ). In summary, the Nadia platform represents a promising and fexible technology for future transcriptomic studies, and other related applications, at cell resolution. Single cell transcriptome profling has recently emerged as a breakthrough technology for understanding how cellular heterogeneity contributes to complex biological systems. Indeed, cultured cells, microorganisms, biopsies, blood and other tissues can be rapidly profled for quantifcation of gene expression at cell resolution. -

Distinct Roles of Jun : Fos and Jun : ATF Dimers in Oncogenesis
Oncogene (2001) 20, 2453 ± 2464 ã 2001 Nature Publishing Group All rights reserved 0950 ± 9232/01 $15.00 www.nature.com/onc Distinct roles of Jun : Fos and Jun : ATF dimers in oncogenesis Hans van Dam*,1 and Marc Castellazzi2 1Department of Molecular Cell Biology, Leiden University Medical Center, Sylvius Laboratories, PO Box 9503, 2300 RA Leiden, The Netherlands; 2Unite de Virologie Humaine, Institut National de la Sante et de la Recherche MeÂdicale (INSERM-U412), Ecole Normale SupeÂrieure, 46 alleÂe d'Italie, 69364 Lyon Cedex 07, France Jun : Fos and Jun : ATF complexes represent two classes dimers with emphasis on their roles in oncogenic of AP-1 dimers that (1) preferentially bind to either transformation in avian model systems. Previous heptameric or octameric AP-1 binding sites, and (2) are reviews on AP-1 and cell transformation include dierently regulated by cellular signaling pathways and references: (Angel and Karin, 1991; Wisdom, 1999; oncogene products. To discriminate between the func- Vogt, 1994; Karin et al., 1997; van Dam and van der tions of Jun : Fos, Jun: ATF and Jun : Jun, mutants were Eb, 1994; Hagmeyer et al., 1995). developed that restrict the ability of Jun to dimerize either to itself, or to Fos(-like) or ATF(-like) partners. Introduction of these mutants in chicken embryo Jun : Fos and Jun : ATF transcription factors: dimeric ®broblasts shows that Jun : Fra2 and Jun : ATF2 dimers complexes with variable composition and activities play distinct, complementary roles in in vitro oncogenesis by inducing either anchorage independence or growth AP-1 sub-units: members of the bZip protein family factor independence, respectively. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Atf1 Arid3a Atf2
TFs Datasets Sequence logos of information models showing cofactor motifs Top 500 peaks (FOXA motif) HepG2, Stanford ARID3A (optimal IDR thresholded) Top 5000 peaks (FOXA motif) All 14818 peaks (IRF motif) K562, HMS ATF1 (optimal IDR thresholded) Top 2500 peaks (SP motif) H1-hESC, HAIB ATF2 Protocol: v042211.1 (optimal IDR thresholded) All 1860 peaks (USF motif) GM12878, HAIB Protocol: PCR1x (SPP obtained from Factorbook) All 1670 peaks (SP motif) GM12878, HAIB Protocol: PCR1x (optimal IDR thresholded) 1550 peaks (USF motif), after masking the SP motif All 4803 peaks (SP motif) H1-hESC, HAIB Protocol: v041610.2 (optimal IDR thresholded) 4697 peaks (USF motif), after masking the SP motif ATF3 All 3284 peaks (SP motif) HepG2, HAIB Protocol: v041610.1 (optimal IDR thresholded) 3209 peaks (USF motif), after masking the SP motif All 11098 peaks (SP motif) K562, HAIB, Replicate 1 Protocol: v041610.1 (raw peaks) All 1232 peaks (SP motif) K562, HMS (optimal IDR thresholded) 1210 peaks (USF motif), after masking the SP motif All 4448 peaks (AP1 motif) BAF155/ HeLa-S3, Stanford SMARCC1 (SPP obtained from Factorbook) 1342 peaks (AP1 motif), after using masking BAF170/ HeLa-S3, Stanford SMARCC2 (SPP obtained from Factorbook) All 17809 peaks (IRF motif) GM12878, HAIB Protocol: PCR1x (optimal IDR thresholded) 17321 peaks (RUNX motif), after masking the IRF motif BCL11A All 2513 peaks (SOX2-OCT4 motif) H1-hESC, HAIB Protocol: PCR1x (optimal IDR thresholded) All 7707 peaks (AP1 motif), after masking noise A549, HAIB Protocol: v042211.1 BCL3 Treatment: -
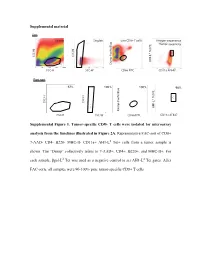
Supplemental Material Supplemental Figure 1. Tumor-Specific CD8+
Supplemental material Sort Scatter Singlets Live CD8+ T cells Antigen-experience Tumor-specifcity c Blue f Tet PE Tet d SSC-H SSC-H AH1-L Dump Paci FSC-H SSC-W CD8α FITC CD11a AF647 Post sort 67% 100%67% 100% 98% c Blue f Tet PE Tet d SSC-H SSC-H AH1-L SSC-Height Dump Paci FSC-H SSC-W CD8α FITC CD11a AF647 SSC-Height Supplemental Figure 1. Tumor-specific CD8+ T cells were isolated for microarray analysis from the timelines illustrated in Figure 2A. Representative FAC-sort of CD8+ 7-AAD- CD4- B220- MHC-II- CD11a+ AH1-Ld Tet+ cells from a tumor sample is shown. The “Dump” collectively refers to 7-AAD+, CD4+, B220+, and MHC-II+. For each sample, βgal-Ld Tet was used as a negative control to set AH1-Ld Tet gates. After FAC-sorts, all samples were 90-100% pure tumor-specific CD8+ T cells. Supplemental Table 1. Transcriptional profiles of published T cell programs compared to tumor-specific TIL T cell program of interest Exhausted (overexpressed) Exhausted (underrexpressed) Memory (overexpressed) Memory (underexpressed) Effector (overexpressed) Effector (underexpressed) Naïve virus-specific CD8+ T Naïve virus-specific CD8+ T Naïve virus-specific CD8+ Exhausted virus-specific CD8+ cells; also not in memory and Memory virus-specific CD8+ cells; also not in exhausted Effector virus-specific T cells; als not in memory, T cell programs compared for T cells vs. effector vs naïve virus-specific T cells vs. and effector vs naïve virus- CD8+ T cells vs. and exhausted vs naïve relative gene expression CD8+ T cells specific CD8+ T cells virus-specific CD8+ T cells Naïve virus-specific CD8+ T Naïve virus-specific CD8+ T Naïve virus-specific CD8+ T Naïve virus-specific CD8+ T Naïve virus-specific CD8+ Naïve virus-specific CD8+ cells cells cells cells T cells T cells Reference Wherry EJ et al. -

ABBOTT MOLECULAR ONCOLOGY and GENETICS 2015-2016 Product Catalog
DESCRIPTOR, 9/12, ALL CAPS ABBOTT MOLECULAR ONCOLOGY AND GENETICS 2015-2016 Product Catalog Area for placed imagery Only use imagery that is relevant to the communication CHOOSE TRANSFORMATION See where it will take you at AbbottMolecular.com 2 Please note some products may not be for sale in all markets. Contact your local representative for availability. ASR (Analyte Specific Reagent) Analytical and performance characteristics are not established CE (CE Marked) Conformité Européenne GPR (General Purpose Reagent) For Laboratory use RUO (Research Use Only) Not for use in diagnostic procedures All products manufactured and/or distributed by Abbott Molecular should be used in accordance with the products’ labeled intended use. Products labeled “Research Use Only” should be used for research applications, and are not for use in diagnostic procedures. CEP, LSI, AneuVysion, MultiVysion, PathVysion and Vysis are registered trademarks of Vysis, Inc., AutoVysion, ProbeChek, SpectrumAqua, SpectrumBlue, SpectrumGreen, SpectrumGold, SpectrumOrange, SpectrumRed, TelVysion, ToTelVysion, UroVysion and VP 2000 are trademarks of Abbott Molecular in various jurisdictions. All other trademarks are the property of their respective owners. Please note some products may not be for sale in all markets. Contact your local representative for availability. 3 Abbott Molecular is Transforming Laboratory Partnerships and Productivity—Today and into the Future As a leader in molecular Our commitment to exploring new clinical frontiers is evident in the development and delivery of innovative diagnostics, Abbott is committed systems and assay solutions that aid physicians in the diagnosis of disease, selection of therapies and monitoring to providing solution-oriented of disease. offerings built on FISH and PCR. -

CREB/PKA Sensitive Signalling Pathways Activate and Maintain
1309 HEPATITIS CREB/PKA sensitive signalling pathways activate Gut: first published as 10.1136/gut.2005.065086 on 4 May 2005. Downloaded from and maintain expression levels of the hepatitis B virus pre-S2/S promoter F Tacke, C Liedtke, S Bocklage, M P Manns, C Trautwein ............................................................................................................................... Gut 2005;54:1309–1317. doi: 10.1136/gut.2005.065086 Background and aims: CREB (cAMP response element binding protein) transcription factors are key regulators of homeostatic functions in the liver, and CRE binding is increased in hepatic inflammation. See end of article for During chronic hepatitis B virus (HBV) infection, mutations or deletions in the pre-S region are frequently authors’ affiliations observed. These mutations can affect the pre-S2/S promoter controlling HBV envelope protein expression ....................... (hepatitis B surface antigen (HBsAg)) and have been associated with worsened clinical outcome. We Correspondence to: aimed to test if CREB activation impacts on HBsAg expression. Dr C Trautwein, Hannover Methods: The effect of the CREB inducer protein kinase A (PKA) was tested by coexpression with HBV wild- Medical School, Department of type vector in vitro. Luciferase reporter gene constructs were cloned to identify novel regulatory regions for Gastroenterology, the HBV pre-S2/S promoter. Electrophoretic mobility shift assay (EMSA) gelshift and supershift Hepatology, and experiments were conducted to confirm DNA transcription factor binding. Endocrinology, Carl- Neuberg-Strasse 1, Results: Coexpression of HBV and PKA resulted in HBV-S mRNA induction and enhanced small envelope D-30625 Hannover, protein expression. We identified a CREB binding motif in the transcribed part of the pre-S2 region, Germany; contributing to basal S promoter activity via binding of activating transcription factor 2 (ATF2). -

Xo PANEL DNA GENE LIST
xO PANEL DNA GENE LIST ~1700 gene comprehensive cancer panel enriched for clinically actionable genes with additional biologically relevant genes (at 400 -500x average coverage on tumor) Genes A-C Genes D-F Genes G-I Genes J-L AATK ATAD2B BTG1 CDH7 CREM DACH1 EPHA1 FES G6PC3 HGF IL18RAP JADE1 LMO1 ABCA1 ATF1 BTG2 CDK1 CRHR1 DACH2 EPHA2 FEV G6PD HIF1A IL1R1 JAK1 LMO2 ABCB1 ATM BTG3 CDK10 CRK DAXX EPHA3 FGF1 GAB1 HIF1AN IL1R2 JAK2 LMO7 ABCB11 ATR BTK CDK11A CRKL DBH EPHA4 FGF10 GAB2 HIST1H1E IL1RAP JAK3 LMTK2 ABCB4 ATRX BTRC CDK11B CRLF2 DCC EPHA5 FGF11 GABPA HIST1H3B IL20RA JARID2 LMTK3 ABCC1 AURKA BUB1 CDK12 CRTC1 DCUN1D1 EPHA6 FGF12 GALNT12 HIST1H4E IL20RB JAZF1 LPHN2 ABCC2 AURKB BUB1B CDK13 CRTC2 DCUN1D2 EPHA7 FGF13 GATA1 HLA-A IL21R JMJD1C LPHN3 ABCG1 AURKC BUB3 CDK14 CRTC3 DDB2 EPHA8 FGF14 GATA2 HLA-B IL22RA1 JMJD4 LPP ABCG2 AXIN1 C11orf30 CDK15 CSF1 DDIT3 EPHB1 FGF16 GATA3 HLF IL22RA2 JMJD6 LRP1B ABI1 AXIN2 CACNA1C CDK16 CSF1R DDR1 EPHB2 FGF17 GATA5 HLTF IL23R JMJD7 LRP5 ABL1 AXL CACNA1S CDK17 CSF2RA DDR2 EPHB3 FGF18 GATA6 HMGA1 IL2RA JMJD8 LRP6 ABL2 B2M CACNB2 CDK18 CSF2RB DDX3X EPHB4 FGF19 GDNF HMGA2 IL2RB JUN LRRK2 ACE BABAM1 CADM2 CDK19 CSF3R DDX5 EPHB6 FGF2 GFI1 HMGCR IL2RG JUNB LSM1 ACSL6 BACH1 CALR CDK2 CSK DDX6 EPOR FGF20 GFI1B HNF1A IL3 JUND LTK ACTA2 BACH2 CAMTA1 CDK20 CSNK1D DEK ERBB2 FGF21 GFRA4 HNF1B IL3RA JUP LYL1 ACTC1 BAG4 CAPRIN2 CDK3 CSNK1E DHFR ERBB3 FGF22 GGCX HNRNPA3 IL4R KAT2A LYN ACVR1 BAI3 CARD10 CDK4 CTCF DHH ERBB4 FGF23 GHR HOXA10 IL5RA KAT2B LZTR1 ACVR1B BAP1 CARD11 CDK5 CTCFL DIAPH1 ERCC1 FGF3 GID4 HOXA11 -

A Membrane-Tethered Transcription Factor Defines a Branch of the Heat Stress Response in Arabidopsis Thaliana
A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana Hongbo Gao*, Federica Brandizzi*†, Christoph Benning‡, and Robert M. Larkin*‡§ *Michigan State University–Department of Energy Plant Research Laboratory, ‡Department of Biochemistry and Molecular Biology, and †Department of Plant Biology, Michigan State University, East Lansing, MI 48824 Communicated by Michael F. Thomashow, Michigan State University, East Lansing, MI, August 28, 2008 (received for review December 14, 2007) In plants, heat stress responses are controlled by heat stress defense responses (13, 14) was recently shown to be heat transcription factors that are conserved among all eukaryotes and inducible and to contribute to heat tolerance (15). These findings can be constitutively expressed or induced by heat. Heat-inducible give evidence of cross-talk between heat stress and other stress transcription factors that are distinct from the ‘‘classical’’ heat signaling pathways. stress transcription factors have also been reported to contribute Membrane-tethered transcription factors (MTTFs) are main- to heat tolerance. Here, we show that bZIP28, a gene encoding a tained in an inactive state by associating with membranes putative membrane-tethered transcription factor, is up-regulated through one or more transmembrane domains (TMDs). In in response to heat and that a bZIP28 null mutant has a striking response to specific signals, an MTTF fragment that contains the heat-sensitive phenotype. The heat-inducible expression of genes transcription factor domain but lacks a TMD, is released from that encode BiP2, an endoplasmic reticulum (ER) chaperone, and membranes by regulated intramembrane proteolysis (RIP), is HSP26.5-P, a small heat shock protein, is attenuated in the bZIP28 redistributed to the nucleus, and regulates the expression of null mutant. -

Uncovering Potential Roles of Differentially Expressed Genes
diagnostics Article Uncovering Potential Roles of Differentially Expressed Genes, Upstream Regulators, and Canonical Pathways in Endometriosis Using an In Silico Genomics Approach Zeenat Mirza 1,2,* and Umama A. Abdel-dayem 1,2 1 King Fahd Medical Research Center, King Abdulaziz University, Jeddah 21589, Saudi Arabia; [email protected] 2 Department of Medical Lab Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah 21589, Saudi Arabia * Correspondence: [email protected] Received: 9 May 2020; Accepted: 17 June 2020; Published: 19 June 2020 Abstract: Endometriosis is characterized by ectopic endometrial tissue implantation, mostly within the peritoneum, and affects women in their reproductive age. Studies have been done to clarify its etiology, but the precise molecular mechanisms and pathophysiology remain unclear. We downloaded genome-wide mRNA expression and clinicopathological data of endometriosis patients and controls from NCBI’s Gene Expression Omnibus, after a systematic search of multiple independent studies comprising 156 endometriosis patients and 118 controls to identify causative genes, risk factors, and potential diagnostic/therapeutic biomarkers. Comprehensive gene expression meta-analysis, pathway analysis, and gene ontology analysis was done using a bioinformatics-based approach. We identified 1590 unique differentially expressed genes (129 upregulated and 1461 downregulated) mapped by IPA as biologically relevant. The top upregulated genes were FOS, EGR1, ZFP36, JUNB, APOD, CST1, GPX3, and PER1, and the top downregulated ones were DIO2, CPM, OLFM4, PALLD, BAG5, TOP2A, PKP4, CDC20B, and SNTN. The most perturbed canonical pathways were mitotic roles of Polo-like kinase, role of Checkpoint kinase proteins in cell cycle checkpoint control, and ATM signaling. Protein–protein interaction analysis showed a strong network association among FOS, EGR1, ZFP36, and JUNB. -

Chapter 1 Introduction: CREB Regulation of Eukaryotic Gene Expression
1 Chapter 1 Introduction: CREB Regulation of Eukaryotic Gene Expression The control of gene expression has evolved to respond to the environmental and intracellular cues that influence cell growth and survival. Key to this control is the ability of cells to affect the activity of transcription factors. Levels of control brought to bear on transcription factors include DNA-binding specificity, post-translational modifications, cis/trans DNA-binding elements and interaction with co-repressors, co-activators and other transcription factors (Fig. 1-1). These modes of regulation provide cells with the capacity to respond with exquisite speed and accuracy to differentiate between the myriad environmental, intercellular and intracellular cues in a context-dependent manner. In multicellular organisms, transactivation of the transcription factor CREB is required for cell survival in neurons and pancreatic -cells [1-5], the development of cell-type specific functions such as control of glucose and lipid metabolism in hepatic cells [6, 7] and the consolidation of long-term memory [1, 8, 9]. The diverse array of stimuli and accompanying kinase cascades that lead to CREB activation made it an ideal subject for investigation as a target for O-GlcNAc glycosylation, a unique form of intracellular and dynamic form of glycosylation. Given its placement at the epicenter of many signaling pathways, we posited it likely that additional layers of control, such as undiscovered post- translational modifications, might exist to regulate CREB activity. 2 ATF/CREB Family of bZIP Transcription Factors. Transcription factors of the basic leucine zipper (bZIP) super family are conserved from S. cerevisiae to mammals. A number of bZIP transcription factors are critical to cellular function, including c-Fos, c- Jun (which together are known as AP-1), C/EBP and CREB.