Entropy and Gibbs Free Energy Worksheet Answers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump Scott M
Purdue University Purdue e-Pubs CTRC Research Publications Cooling Technologies Research Center 2012 Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump Scott M. Flueckiger Purdue University Fabien Volle Laboratoire des Sciences des Procédés et des Matériaux S V. Garimella Purdue University, [email protected] Rajiv K. Mongia Intel Corporation Follow this and additional works at: http://docs.lib.purdue.edu/coolingpubs Flueckiger, Scott M.; Volle, Fabien; Garimella, S V.; and Mongia, Rajiv K., "Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump" (2012). CTRC Research Publications. Paper 182. http://dx.doi.org/http://dx.doi.org/10.1016/j.enconman.2012.04.015 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump* Scott M. Flueckiger1, Fabien Volle2, Suresh V. Garimella1**, Rajiv K. Mongia3 1 Cooling Technologies Research Center, an NSF I/UCRC School of Mechanical Engineering and Birck Nanotechnology Center 585 Purdue Mall, Purdue University West Lafayette, Indiana 47907-2088 USA 2 Laboratoire des Sciences des Procédés et des Matériaux (LSPM, UPR 3407 CNRS), Université Paris XIII, 99 avenue J. B. Clément, 93430 Villetaneuse, France 3 Intel Corporation Santa Clara, California 95054 USA * Submitted for publication in Energy Conversion and Management ** Author to who correspondence should be addressed: (765) 494-5621, [email protected] Abstract Representative reversible endothermic chemical reactions (paraldehyde depolymerization and 2-proponal dehydrogenation) are theoretically assessed for their use in a chemical heat pump design for compact thermal management applications. -

Chapter 3. Second and Third Law of Thermodynamics
Chapter 3. Second and third law of thermodynamics Important Concepts Review Entropy; Gibbs Free Energy • Entropy (S) – definitions Law of Corresponding States (ch 1 notes) • Entropy changes in reversible and Reduced pressure, temperatures, volumes irreversible processes • Entropy of mixing of ideal gases • 2nd law of thermodynamics • 3rd law of thermodynamics Math • Free energy Numerical integration by computer • Maxwell relations (Trapezoidal integration • Dependence of free energy on P, V, T https://en.wikipedia.org/wiki/Trapezoidal_rule) • Thermodynamic functions of mixtures Properties of partial differential equations • Partial molar quantities and chemical Rules for inequalities potential Major Concept Review • Adiabats vs. isotherms p1V1 p2V2 • Sign convention for work and heat w done on c=C /R vm system, q supplied to system : + p1V1 p2V2 =Cp/CV w done by system, q removed from system : c c V1T1 V2T2 - • Joule-Thomson expansion (DH=0); • State variables depend on final & initial state; not Joule-Thomson coefficient, inversion path. temperature • Reversible change occurs in series of equilibrium V states T TT V P p • Adiabatic q = 0; Isothermal DT = 0 H CP • Equations of state for enthalpy, H and internal • Formation reaction; enthalpies of energy, U reaction, Hess’s Law; other changes D rxn H iD f Hi i T D rxn H Drxn Href DrxnCpdT Tref • Calorimetry Spontaneous and Nonspontaneous Changes First Law: when one form of energy is converted to another, the total energy in universe is conserved. • Does not give any other restriction on a process • But many processes have a natural direction Examples • gas expands into a vacuum; not the reverse • can burn paper; can't unburn paper • heat never flows spontaneously from cold to hot These changes are called nonspontaneous changes. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

Chemical Reactions Involve Energy Changes
Page 1 of 6 KEY CONCEPT Chemical reactions involve energy changes. BEFORE, you learned NOW, you will learn • Bonds are broken and made • About the energy in chemical during chemical reactions bonds between atoms • Mass is conserved in all • Why some chemical reactions chemical reactions release energy • Chemical reactions are • Why some chemical reactions represented by balanced absorb energy chemical equations VOCABULARY EXPLORE Energy Changes bond energy p. 86 How can you identify a transfer of energy? exothermic reaction p. 87 endothermic reaction p. 87 PROCEDURE MATERIALS photosynthesis p. 90 • graduated cylinder 1 Pour 50 ml of hot tap water into the cup • hot tap water and place the thermometer in the cup. • plastic cup 2 Wait 30 seconds, then record the • thermometer temperature of the water. • stopwatch • plastic spoon 3 Measure 5 tsp of Epsom salts. Add the Epsom salts to the beaker and immedi- • Epsom salts ately record the temperature while stirring the contents of the cup. 4 Continue to record the temperature every 30 seconds for 2 minutes. WHAT DO YOU THINK? • What happened to the temperature after you added the Epsom salts? • What do you think caused this change to occur? Chemical reactions release or absorb energy. COMBINATION NOTES Chemical reactions involve breaking bonds in reactants and forming Use combination notes new bonds in products. Breaking bonds requires energy, and forming to organize information on how chemical reactions bonds releases energy. The energy associated with bonds is called bond absorb or release energy. energy. What happens to this energy during a chemical reaction? Chemists have determined the bond energy for bonds between atoms. -
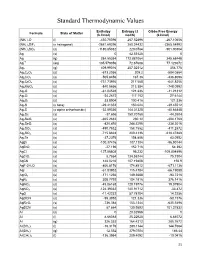
Standard Thermodynamic Values
Standard Thermodynamic Values Enthalpy Entropy (J Gibbs Free Energy Formula State of Matter (kJ/mol) mol/K) (kJ/mol) (NH4)2O (l) -430.70096 267.52496 -267.10656 (NH4)2SiF6 (s hexagonal) -2681.69296 280.24432 -2365.54992 (NH4)2SO4 (s) -1180.85032 220.0784 -901.90304 Ag (s) 0 42.55128 0 Ag (g) 284.55384 172.887064 245.68448 Ag+1 (aq) 105.579056 72.67608 77.123672 Ag2 (g) 409.99016 257.02312 358.778 Ag2C2O4 (s) -673.2056 209.2 -584.0864 Ag2CO3 (s) -505.8456 167.36 -436.8096 Ag2CrO4 (s) -731.73976 217.568 -641.8256 Ag2MoO4 (s) -840.5656 213.384 -748.0992 Ag2O (s) -31.04528 121.336 -11.21312 Ag2O2 (s) -24.2672 117.152 27.6144 Ag2O3 (s) 33.8904 100.416 121.336 Ag2S (s beta) -29.41352 150.624 -39.45512 Ag2S (s alpha orthorhombic) -32.59336 144.01328 -40.66848 Ag2Se (s) -37.656 150.70768 -44.3504 Ag2SeO3 (s) -365.2632 230.12 -304.1768 Ag2SeO4 (s) -420.492 248.5296 -334.3016 Ag2SO3 (s) -490.7832 158.1552 -411.2872 Ag2SO4 (s) -715.8824 200.4136 -618.47888 Ag2Te (s) -37.2376 154.808 43.0952 AgBr (s) -100.37416 107.1104 -96.90144 AgBrO3 (s) -27.196 152.716 54.392 AgCl (s) -127.06808 96.232 -109.804896 AgClO2 (s) 8.7864 134.55744 75.7304 AgCN (s) 146.0216 107.19408 156.9 AgF•2H2O (s) -800.8176 174.8912 -671.1136 AgI (s) -61.83952 115.4784 -66.19088 AgIO3 (s) -171.1256 149.3688 -93.7216 AgN3 (s) 308.7792 104.1816 376.1416 AgNO2 (s) -45.06168 128.19776 19.07904 AgNO3 (s) -124.39032 140.91712 -33.472 AgO (s) -11.42232 57.78104 14.2256 AgOCN (s) -95.3952 121.336 -58.1576 AgReO4 (s) -736.384 153.1344 -635.5496 AgSCN (s) 87.864 130.9592 101.37832 Al (s) -

Energy and Enthalpy Thermodynamics
Energy and Energy and Enthalpy Thermodynamics The internal energy (E) of a system consists of The energy change of a reaction the kinetic energy of all the particles (related to is measured at constant temperature) plus the potential energy of volume (in a bomb interaction between the particles and within the calorimeter). particles (eg bonding). We can only measure the change in energy of the system (units = J or Nm). More conveniently reactions are performed at constant Energy pressure which measures enthalpy change, ∆H. initial state final state ∆H ~ ∆E for most reactions we study. final state initial state ∆H < 0 exothermic reaction Energy "lost" to surroundings Energy "gained" from surroundings ∆H > 0 endothermic reaction < 0 > 0 2 o Enthalpy of formation, fH Hess’s Law o Hess's Law: The heat change in any reaction is the The standard enthalpy of formation, fH , is the change in enthalpy when one mole of a substance is formed from same whether the reaction takes place in one step or its elements under a standard pressure of 1 atm. several steps, i.e. the overall energy change of a reaction is independent of the route taken. The heat of formation of any element in its standard state is defined as zero. o The standard enthalpy of reaction, H , is the sum of the enthalpy of the products minus the sum of the enthalpy of the reactants. Start End o o o H = prod nfH - react nfH 3 4 Example Application – energy foods! Calculate Ho for CH (g) + 2O (g) CO (g) + 2H O(l) Do you get more energy from the metabolism of 1.0 g of sugar or -

Lecture 7: "Basics of Star Formation and Stellar Nucleosynthesis" Outline
Lecture 7: "Basics of Star Formation and Stellar Nucleosynthesis" Outline 1. Formation of elements in stars 2. Injection of new elements into ISM 3. Phases of star-formation 4. Evolution of stars Mark Whittle University of Virginia Life Cycle of Matter in Milky Way Molecular clouds New clouds with gravitationally collapse heavier composition to form stellar clusters of stars are formed Molecular cloud Stars synthesize Most massive stars evolve He, C, Si, Fe via quickly and die as supernovae – nucleosynthesis heavier elements are injected in space Solar abundances • Observation of atomic absorption lines in the solar spectrum • For some (heavy) elements meteoritic data are used Solar abundance pattern: • Regularities reflect nuclear properties • Several different processes • Mixture of material from many, many stars 5 SolarNucleosynthesis abundances: key facts • Solar• Decreaseabundance in abundance pattern: with atomic number: - Large negative anomaly at Be, B, Li • Regularities reflect nuclear properties - Moderate positive anomaly around Fe • Several different processes 6 - Sawtooth pattern from odd-even effect • Mixture of material from many, many stars Origin of elements • The Big Bang: H, D, 3,4He, Li • All other nuclei were synthesized in stars • Stellar nucleosynthesis ⇔ 3 key processes: - Nuclear fusion: PP cycles, CNO bi-cycle, He burning, C burning, O burning, Si burning ⇒ till 40Ca - Photodisintegration rearrangement: Intense gamma-ray radiation drives nuclear rearrangement ⇒ 56Fe - Most nuclei heavier than 56Fe are due to neutron -

Chemistry 130 Gibbs Free Energy
Chemistry 130 Gibbs Free Energy Dr. John F. C. Turner 409 Buehler Hall [email protected] Chemistry 130 Equilibrium and energy So far in chemistry 130, and in Chemistry 120, we have described chemical reactions thermodynamically by using U - the change in internal energy, U, which involves heat transferring in or out of the system only or H - the change in enthalpy, H, which involves heat transfers in and out of the system as well as changes in work. U applies at constant volume, where as H applies at constant pressure. Chemistry 130 Equilibrium and energy When chemical systems change, either physically through melting, evaporation, freezing or some other physical process variables (V, P, T) or chemically by reaction variables (ni) they move to a point of equilibrium by either exothermic or endothermic processes. Characterizing the change as exothermic or endothermic does not tell us whether the change is spontaneous or not. Both endothermic and exothermic processes are seen to occur spontaneously. Chemistry 130 Equilibrium and energy Our descriptions of reactions and other chemical changes are on the basis of exothermicity or endothermicity ± whether H is negative or positive H is negative ± exothermic H is positive ± endothermic As a description of changes in heat content and work, these are adequate but they do not describe whether a process is spontaneous or not. There are endothermic processes that are spontaneous ± evaporation of water, the dissolution of ammonium chloride in water, the melting of ice and so on. We need a thermodynamic description of spontaneous processes in order to fully describe a chemical system Chemistry 130 Equilibrium and energy A spontaneous process is one that takes place without any influence external to the system. -

Lecture 13. Thermodynamic Potentials (Ch
Lecture 13. Thermodynamic Potentials (Ch. 5) So far, we have been using the total internal energy U and, sometimes, the enthalpy H to characterize various macroscopic systems. These functions are called the thermodynamic potentials: all the thermodynamic properties of the system can be found by taking partial derivatives of the TP. For each TP, a set of so-called “natural variables” exists: −= + μ NddVPSdTUd = + + μ NddPVSdTHd Today we’ll introduce the other two thermodynamic potentials: theHelmhotzfree energy F and Gibbs free energy G. Depending on the type of a process, one of these four thermodynamic potentials provides the most convenient description (and is tabulated). All four functions have units of energy. When considering different types of Potential Variables processes, we will be interested in two main U (S,V,N) S, V, N issues: H (S,P,N) S, P, N what determines the stability of a system and how the system evolves towards an F (T,V,N) V, T, N equilibrium; G (T,P,N) P, T, N how much work can be extracted from a system. Diffusive Equilibrium and Chemical Potential For completeness, let’s rcall what we’vee learned about the chemical potential. 1 P μ d U T d= S− P+ μ dVd d= S Nd U +dV d − N T T T The meaning of the partial derivative (∂S/∂N)U,V : let’s fix VA and VB (the membrane’s position is fixed), but U , V , S U , V , S A A A B B B assume that the membrane becomes permeable for gas molecules (exchange of both U and N between the sub- ns ilesystems, the molecuA and B are the same ). -

Thermodynamic Potentials and Thermodynamic Relations In
arXiv:1004.0337 Thermodynamic potentials and Thermodynamic Relations in Nonextensive Thermodynamics Guo Lina, Du Jiulin Department of Physics, School of Science, Tianjin University, Tianjin 300072, China Abstract The generalized Gibbs free energy and enthalpy is derived in the framework of nonextensive thermodynamics by using the so-called physical temperature and the physical pressure. Some thermodynamical relations are studied by considering the difference between the physical temperature and the inverse of Lagrange multiplier. The thermodynamical relation between the heat capacities at a constant volume and at a constant pressure is obtained using the generalized thermodynamical potential, which is found to be different from the traditional one in Gibbs thermodynamics. But, the expressions for the heat capacities using the generalized thermodynamical potentials are unchanged. PACS: 05.70.-a; 05.20.-y; 05.90.+m Keywords: Nonextensive thermodynamics; The generalized thermodynamical relations 1. Introduction In the traditional thermodynamics, there are several fundamental thermodynamic potentials, such as internal energy U , Helmholtz free energy F , enthalpy H , Gibbs free energyG . Each of them is a function of temperature T , pressure P and volume V . They and their thermodynamical relations constitute the basis of classical thermodynamics. Recently, nonextensive thermo-statitstics has attracted significent interests and has obtained wide applications to so many interesting fields, such as astrophysics [2, 1 3], real gases [4], plasma [5], nuclear reactions [6] and so on. Especially, one has been studying the problems whether the thermodynamic potentials and their thermo- dynamic relations in nonextensive thermodynamics are the same as those in the classical thermodynamics [7, 8]. In this paper, under the framework of nonextensive thermodynamics, we study the generalized Gibbs free energy Gq in section 2, the heat capacity at constant volume CVq and heat capacity at constant pressure CPq in section 3, and the generalized enthalpy H q in Sec.4. -

Endothermic and Exothermic Reactions
Name: ________________________ Date: ____________ Period: ____________ Endothermic and Exothermic Reactions Read the following and take notes in the margins. Respond to questions 1-3 at the end. Let's see what Sam and Julie are up to in the chemistry lab. Excited but a bit confused, Sam and Julie run to their chemistry teacher. Sam asks, “Teacher, why did my flask turn cold after adding the salt to water, while Julie’s flask turned hot?” The teacher replies: “That’s because you were given two different salts. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. Let me first reveal the identity of your salts: Salt A is ammonium nitrate and Salt B is calcium chloride." Now, Sam and Julie are curious about the difference between an endothermic and an exothermic reaction. Consider the reaction mixture—salt plus water—as the system and the flask as the surrounding. In Sam’s case, when ammonium nitrate was dissolved in water, the system absorbed heat from the surrounding, the flask, and thus the flask felt cold. This is an example of an endothermic reaction. In Julie’s case, when calcium chloride was dissolved in water, the system released heat into the surroundings, the flask, and thus the flask felt hot. This is an example of an exothermic reaction. The reaction going on in Sam’s flask can be represented as: NH4NO3 (s) + heat ---> NH4+ (aq) + NO3- (aq) You can see, heat is absorbed during the above reaction, lowering the temperature of the reaction mixture, and thus the reaction flask feels cold. -

Non-Electric Applications of Fusion
Non-Electric Applications of Fusion Final Report to FESAC, July 31, 2003 Executive Summary This report examines the possibility of non-electric applications of fusion. In particular, FESAC was asked to consider “whether the Fusion Energy Sciences program should broaden its scope and activities to include non-electric applications of intermediate-term fusion devices.” During this process, FESAC was asked to consider the following questions: • What are the most promising opportunities for using intermediate-term fusion devices to contribute to the Department of Energy missions beyond the production of electricity? • What steps should the program take to incorporate these opportunities into plans for fusion research? • Are there any possible negative impacts to pursuing these opportunities and are there ways to mitigate these possible impacts? The panel adopted the following three criteria to evaluate all of the non-electric applications considered: 1. Will the application be viewed as necessary to solve a "national problem" or will the application be viewed as a solution by the funding entity? 2. What are the technical requirements on fusion imposed by this application with respect to the present state of fusion and the technical requirements imposed by electricity production? What R&D is required to meet these requirements and is it "on the path" to electricity production? 3. What is the competition for this application, and what is the likelihood that fusion can beat it? It is the opinion of this panel that the most promising opportunities for non-electric applications of fusion fall into four categories: 1. Near-Term Applications 2. Transmutation 3. Hydrogen Production 4.