Solvent Development for CO Absorption Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

B REGULATION (EC) No 1334/2008 of the EUROPEAN
2008R1334 — EN — 13.05.2016 — 011.001 — 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B REGULATION (EC) No 1334/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC (Text with EEA relevance) (OJ L 354, 31.12.2008, p. 34) Amended by: Official Journal No page date ►M1 Commission Implementing Regulation (EU) No 872/2012 of 1 October L 267 1 2.10.2012 2012 ►M2 Commission Regulation (EU) No 545/2013 of 14 June 2013 L 163 15 15.6.2013 ►M3 Commission Regulation (EU) No 985/2013 of 14 October 2013 L 273 18 15.10.2013 ►M4 Commission Regulation (EU) No 246/2014 of 13 March 2014 L 74 58 14.3.2014 ►M5 Commission Regulation (EU) No 1098/2014 of 17 October 2014 L 300 41 18.10.2014 ►M6 Commission Regulation (EU) 2015/648 of 24 April 2015 L 107 15 25.4.2015 ►M7 Commission Regulation (EU) 2015/1102 of 8 July 2015 L 181 54 9.7.2015 ►M8 Commission Regulation (EU) 2015/1760 of 1 October 2015 L 257 27 2.10.2015 ►M9 Commission Regulation (EU) 2016/54 of 19 January 2016 L 13 40 20.1.2016 ►M10 Commission Regulation (EU) 2016/55 of 19 January 2016 L 13 43 20.1.2016 ►M11 Commission Regulation (EU) 2016/178 of 10 February 2016 L 35 6 11.2.2016 ►M12 Commission Regulation (EU) 2016/637 of 22 April 2016 L 108 24 23.4.2016 2008R1334 — EN — 13.05.2016 — 011.001 -

United States Patent Office Patented Nov
3,215,732 United States Patent Office Patented Nov. 2, 1965 1. 2 example isopropanol. The reduction product may be 3,215,732 reacted with the said amine in the presence of a diluent NAPHTHALENE DERVATIVES or solvent for example ethanol and the process may be John S. Stephenson, Taplow, England, assignor to Im accelerated or completed by the application of heat. perial Chemical industries Limited, London, England, It is to be understood that the said reduction product a corporation of Great Britain No Drawing. Fied Apr. 26, 1961, Ser. No. 105,571. is believed to be one or other of the two naphthalene Claims priority, application Great Britain, May 4, 1960, derivatives of the formula: 15,716/60; Oct. 7, 1960, 34,438/60 CHOICH2X 9 Caims. (C. 260-501) 10 This invention relates to organic compounds and more Y particularly it relates to new naphthalene derivatives which possess valuable therapeutic properties. According to the invention I provide naphthalene and 15 derivatives of the formula: O CHOECEINR 1Ri of SCH, Y Y 20 NX, Z. wherein R stands for hydrogen or for a metay1 lauva. wherein R1 stands for hydrogen or for a methyl radical, R2 stands for a branched chain alkyl radical of not a mixture thereof, and either of these compounds or a more than 4 carbon atoms and Y and Z stand for hy mixture thereof can be used as starting material for the drogen, halogen, lower alkyl and lower alkoxy, and the 25 manufacture of the compounds of the present invention. non-toxic, pharmaceutically-acceptable salts thereof. -

Putrescine and Cadaverine in Thehuman
Gut 1993; 34:489-493 489 Presence ofN-acyl and acetoxy derivatives of putrescine and cadaverine in the human gut Gut: first published as 10.1136/gut.34.4.489 on 1 April 1993. Downloaded from K E Murray, K J Shaw, R F Adams, P L Conway Abstract from isolates of gut micro-organisms. Identifica- N-acyl and acetoxy derivatives of putrescine tion of the compounds was obtained in 1-10 .tg and cadaverine have been found in the faeces samples by thin layer chromatography (TLC), of children and in cultures of isolates of gut FDMS, and accurate mass measurement of the bacteria. The evidence was accumulated from molecular ions of the 1-dimethylaminonaphtha- two dimensional, thin layer chromatography, lene-5-sulphonyl- (DANS) derivatives of the field desorption mass spectrometry, and amines concerned. accurate mass measurement of the DANS derivatives of the amines. The acetoxy com- pounds of putrescine and cadaverine have not Methods previously been reported (Gut 1993; 34: 489-493) SUBJECTS AND SOURCES OF SPECIMENS An initial source of faecal specimens was a group of five infants admitted to hospital with salmon- Monoacetylcadaverine (N-acetyl-1,5-diamino- ellosis (Table I). The specimens were taken pentane) and monopropionylcadaverine (N- before antibiotic treatment was begun. A group propionyl-1,5-diaminopentane) have been of 13 children (reported previously6) provided shown to occur frequently in the urine of a second source of faecal specimens. These schizophrenic patients, of psychotic or mentally children had been admitted to hospital for defective patients, and of mentally normal reasons unrelated to gastric problems and were patients in hospital.' These same compounds not receiving any drug treatment. -

Bssl V11.0.Pdf
Content 1 Introduction 3 2 Definitions 3 3 Testing methods 7 4 Scope and validity 8 5 Consumer safety limits 9 Annex I Compilation of single substances Annex II Usage Ranges bluesign® system substances list (BSSL) | v11.0 | December 1, 2020 ©bluesign technologies ag | www.bluesign.com 2 1 Introduction The document specifies the limits for chemical substances in articles. It also defines usage bans for chemical substances prohibited from the manufacturing of articles. It is important to know that due to quantity and range of listed substances and substance groups the consumer safety limits cannot be controlled by testing of articles alone and/or by confirmation declarations from suppliers (conventional RSL and/or testing approach). This is the reason why the bluesign® SYSTEM integrates the up-stream parts of the manufacturing chain including chemical suppliers. Only an input-stream management with an appropriate network of bluesign® SYSTEM PARTNERS leads to comprehensive knowledge on chemical products and assures that restrictions and bans are achieved. Definitions 2.1 Accessory A component of a consumer product which is not classified as textile fabric (e.g. button, label, zipper, etc.) 2.2 Article An object which during production is given a special shape, surface or design, which determines its function to a greater degree than does its chemical composition (fibers, textile fabrics, buttons, zippers, etc.). 2.3 BSSL bluesign® system substances list (BSSL) Consumer safety limits. A list that specifies consumer safety limits for chemical substances in articles. It also defines usage bans for chemical substances prohibited from the manufacturing of articles. 2.4 CAS CAS registry numbers are unique numerical identifiers for chemical elements, compounds, polymers, biological sequences, mixtures and alloys. -
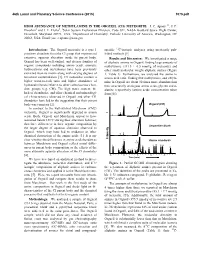
High Abundance of Methylamine in the Orgueil (Ci1) Meteorite
46th Lunar and Planetary Science Conference (2015) 1075.pdf HIGH ABUNDANCE OF METHYLAMINE IN THE ORGUEIL (CI1) METEORITE. J. C. Aponte1,2, J. P. Dworkin1 and J. E. Elsila1, 1Solar System Exploration Division, Code 691, NASA Goddard Space Flight Center, Greenbelt, Maryland 20771, USA, 2Department of Chemistry, Catholic University of America, Washington, DC 20064, USA. Email: [email protected] Introduction: The Orgueil meteorite is a type 1 specific 13C-isotopic analyzes using previously pub- primitive chondrite from the CI group that experienced lished methods [5]. extensive aqueous alteration inside its parent body. Results and Discussion: We investigated a range Orgueil has been well studied, and diverse families of of aliphatic amines in Orgueil, finding large amounts of organic compounds including amino acids, aromatic methylamine (331.5 ± 0.5 nmol/g of meteorite) and hydrocarbons and nucleobases have been previously other small molecular weight aliphatic amines (Figure extracted from its matrix along with varying degrees of 1, Table 1). Furthermore, we analyzed the amine to terrestrial contamination [1]. CI meteorites contain a amino acid ratio, finding that methylamine and ethyla- higher water-to-rock ratio and higher abundance of mine in Orgueil are about 30 times more abundant than hydrated minerals relative to other carbonaceous chon- their structurally analogous amino acids, glycine and α- drite groups (e.g. CM). The high water content, the alanine respectively (amino acids concentration taken lack of chondrules, and other chemical and mineralogi- from [6]). cal characterisitcs observed in Orgueil and other CI1 chondrites have led to the suggestion that their parent body was cometary [2]. -

United States Patent (19) (11) 3,835,191 Wagner Et Al
United States Patent (19) (11) 3,835,191 Wagner et al. (45) Sept. 10, 1974 54 ALDIMNES AND KETIMINES 2,394,530 2/1946 Bruson et al.................... 260/566 R CONTAINING HYDROXYMETHYL GROUPS 3,420,800 1/1969 Haggis............................. 260/566 R AND PREPARATION THEREOF 3,529,023 9/1970 Leshin............................. 260/566 R 75 Inventors: Kuno Wagner; Manfred Hajek, both of Leverkusen, Germany OTHER PUBLICATIONS 73) Assignee: Bayer Aktiengesellschaft Layer, Chemical Reviews, Vol. 63, pg. 492 (1963) 22 Filed: Nov. 26, 1969 Primary Examiner-Leon Zitver (21) Appl. No.: 880,420 Assistant Examiner-Gerald A. Schwartz Attorney, Agent, or Firm-Connolly and Hutz (30 Foreign Application Priority Data Dec. 14, 1968 Germany....................... ... 1814832 57 ABSTRACT 52 U.S. Cl...... 260/566 R, 260/77.5 AT, 260/464, Novel aldimines and ketimines which contain hydroxy 260/465 E, 260/465.5 R, 260/468 H, methyl groups are provided. These new compounds 260/468 J, 260/471 A, 260/482 R are prepared by reacting Schiff's bases which have ac (5ll Int. Cl........................................... C07 c 119/00 tive hydrogen atoms in a-position to the C=N-group 58 Field of Search......... 260/566 R, 468 H, 468 J, with formaldehyde or formaldehyde yielding com 260/471 A, 464, 465 E, 465.5 R, 482 R pounds. The new compounds can, e.g., be used as 56 References Cited plasticizing chain lengthening agents for the prepara UNITED STATES PATENTS tion of polyurethane plastics. 2,000,041 5/1935 Semon et al.................... 260/566 R 11 Claims, No Drawings 3,835,191 1. -

High-Affinity Olfactory Receptor for the Death-Associated Odor Cadaverine
High-affinity olfactory receptor for the death-associated odor cadaverine Ashiq Hussaina,1,2, Luis R. Saraivaa,2,3, David M. Ferrerob,2, Gaurav Ahujaa,2, Venkatesh S. Krishnaa, Stephen D. Liberlesb,4, and Sigrun I. Korschinga,4 aInstitut für Genetik, Universität zu Köln, 50674 Cologne, Germany; and bDepartment of Cell Biology, Harvard University, Boston, MA 02115 Edited* by Cornelia I. Bargmann, The Rockefeller University, New York, NY, and approved October 18, 2013 (received for review October 2, 2013) Carrion smell is strongly repugnant to humans and triggers distinct Results innate behaviors in many other species. This smell is mainly carried Zebrafish Avoid a Cadaverine Odor Source. The zebrafish has in by two small aliphatic diamines, putrescine and cadaverine, which recent years emerged as an important model system for un- are generated by bacterial decarboxylation of the basic amino derstanding olfaction in vertebrates because of a remarkable acids ornithine and lysine. Depending on the species, these diamines similarity in the basic principles of olfactory representation (9) may also serve as feeding attractants, oviposition attractants, or and some technical advantages over the mammalian system (10). social cues. Behavioral responses to diamines have not been in- However, behavioral responses of zebrafish to diamines have not vestigated in zebrafish, a powerful model system for studying been described. We report here that zebrafish, like humans, show vertebrate olfaction. Furthermore, olfactory receptors that detect pronounced innate aversion behavior for cadaverine (Fig. 1). cadaverine and putrescine have not been identified in any species We developed a valence assay to measure behavioral re- so far. Here, we show robust olfactory-mediated avoidance behav- sponses of zebrafish to olfactory cues. -

Rapport En Français
Additifs du 1,1,1-trichloroéthane État de l’art et enjeux en contexte « sites et sols pollués » Rapport final BRGM/RP-70190-FR Décembre 2020 Additifs du 1,1,1-trichloroéthane État de l’art et enjeux en contexte « sites et sols pollués » Rapport final BRGM/RP-70190-FR Décembre 2020 G. Boissard Vérificateur : Approbateur : Nom : Aline Coftier Nom : Sandrine Lemal Fonction : Cheffe de projets Fonction : Responsable Unité Sites Sols Pollués Date : 08/12/2020 Date : 14/12/2020 Signature : Signature : Le système de management de la qualité et de l’environnement est certifié par AFNOR selon les normes ISO 9001 et ISO 14001. Contact : [email protected] Mots-clés : Émergents, Pollution, Solvant, Sites pollués. En bibliographie, ce rapport sera cité de la façon suivante : Boissard G. (2020) - Additifs du 1,1,1-trichloroéthane – État de l’art et enjeux en contexte « sites et sols pollués ». Rapport final. BRGM/RP-70190-FR, 262 p., 4 fig., 23 tab., 12 ann. © BRGM, 2020, ce document ne peut être reproduit en totalité ou en partie sans l’autorisation expresse du BRGM. Additifs du 1,1,1-trichloroéthane - État de l’art et enjeux en contexte « sites et sols pollués » Synthèse Le 1,1,1-trichloroéthane (nommé TCA dans la suite du document) est un composé chloré largement utilisé comme solvant pour le dégraissage des métaux. Comme de nombreux autres solvants chlorés, il est généralement formulé avec des additifs pour augmenter sa stabilité et améliorer ses performances. Le TCA est considéré comme le solvant le moins stable parmi d’autres solvants tels que le trichloroéthylène (TCE), le tétrachloroéthylène (PCE) et le dichlorométhane. -

Flammables CAS DOT SHHC Sources Number Chemical Name
2010 Right to Know Special Health Hazardous Substance List Substance Common Name Flammables CAS DOT SHHC Sources Number Chemical Name 2057 # ACETAL 105-57-7 1088 F3 3 15 17 ETHANE, 1,1-DIETHOXY- 0001 # ACETALDEHYDE 75-07-0 1089 CA MU TE F4 1 2 3 4 5 6 7 8 R2 15 17 18 20 21 22 ACETALDEHYDE 0003 # ACETALDEHYDE OXIME 107-29-9 2332 F3 3 17 ACETALDEHYDE, OXIME 0004 # ACETIC ACID 64-19-7 2789 CO 1 2 3 4 15 17 20 ACETIC ACID 0005 # ACETIC ANHYDRIDE 108-24-7 1715 CO 1 2 3 4 15 17 20 ACETIC ACID, ANHYDRIDE 0006 # ACETONE 67-64-1 1090 F3 1 2 3 4 8 15 17 20 21 2-PROPANONE 0007 # ACETONE CYANOHYDRIN 75-86-5 1541 R2 2 3 4 6 15 17 18 19 20 21 PROPANENITRILE, 2-HYDROXY-2-METHYL- 0008 # ACETONITRILE 75-05-8 1648 F3 1 2 3 4 6 8 15 17 18 20 21 ACETONITRILE 2961 ACETOPHENONE 98-86-2 1993 2 3 6 8 15 17 18 20 21 ETHANONE, 1-PHENYL- Page 1 of 102 2010 Right to Know Special Health Hazardous Substance List Substance Common Name Flammables CAS DOT SHHC Sources Number Chemical Name 0009 # ACETYL ACETONE PEROXIDE 37187-22-7 3105 F3 R3 3 17 2,4-PENTANEDIONE, PEROXIDE 0013 # ACETYL CHLORIDE 75-36-5 1717 CO F3 R2 3 8 15 17 20 21 ACETYL CHLORIDE 0014 ACETYL CYCLOHEXANE SULFONYL PEROXIDE 3179-56-4 3112 3 17 PEROXIDE, ACETYL CYCLOHEXYLSULFONYL 0015 # ACETYLENE 74-86-2 1001 F4 R3 2 3 4 6 15 17 22 ETHYNE 0018 ACETYL METHYL CARBINOL 513-86-0 2621 3 17 2-BUTANONE, 3-HYDROXY- 0019 # ACETYL PEROXIDE 110-22-5 2084 R4 3 15 17 PEROXIDE, DIACETYL 2064 # ACRIDINE 260-94-6 2713 F3 3 17 ACRIDINE 0021 # ACROLEIN 107-02-8 1092 F3 R3 1 2 3 4 6 7 8 14 15 17 18 19 20 2-PROPENAL 21 22 -

Amine Based Solvent for CO2 Absorption “From Molecular Structure to Process”
Amine Based Solvent for CO2 Absorption “From Molecular Structure to Process” Prachi Singh Amine Based Solvent for CO2 Absorption “From Molecular Structure to Process” Prachi Singh Dit proefschrift is goedgekeurd door de promotor: Prof.dr.ir. W. P. M. van Swaaij Samenstelling Promotiecommissie: Voorzitter: Prof.dr. G. van der Steenhoven Universiteit Twente Secretaris: Prof.dr. G. van der Steenhoven Universiteit Twente Promotor: Prof.dr.ir. W. P. M. van Swaaij Universiteit Twente Ass. Promotor: Dr.ir. D. W. F. Brilman Universiteit Twente Referenten: Dr.ir. J. A. Hogendoorn Universiteit Twente Dr.ir. E. Falck da Silva SINTEF, Norway Deskundige: Dr.ir. F. H. Geuzebroek Shell Technology Centre Amsterdam Leden: Prof.dr. S. R. A. Kersten Universiteit Twente Prof.dr.ir. A. Nijmeijer Universiteit Twente Prof.dr.ir. H. J. Heeres Rijksuniversiteit Groningen Dit onderzoek werd financieel ondersteund door nationale projecten CATO Prachi Singh Amine based solvent for CO2 absorption “From molecular structure to process”. Thesis, University of Twente, The Netherlands ISBN 978-90-365-3200-6 Printed by Wöhrmann Print Service Cover Design: Painting “Green horizon” by Prachi Singh Copyright © 2011 by Prachi Singh All rights reserved. NO part of the material protected by this copyright notice may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopy, recording or by any information storage and material system, without written permission from the publisher. AMINE BASED SOLVENT FOR CO2 ABSORPTION “FROM MOLECULAR STRUCTURE TO PROCESS” PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit Twente, op gezag van de rector magnificus, prof.dr. -

The NIOSH Occupational Exposure Banding Process for Chemical Risk Management
TECHNICAL REPORT The NIOSH Occupational Exposure Banding Process for Chemical Risk Management Centers for Disease Control and Prevention National Institute for Occupational Safety and Health This page intentionally blank. Technical Report The NIOSH Occupational Exposure Banding Process for Chemical Risk Management DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health This document is in the public domain and may be freely copied or reprinted. Disclaimer Mention of any company or product does not constitute endorsement by the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. In addition, citations to websites external to NIOSH do not constitute NIOSH endorsement of the sponsor- ing organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these websites. All web addresses referenced in this document were accessible as of the publication date. Get More Information Find NIOSH products and get answers to workplace safety and health questions: 1-800-CDC-INFO (1-800-232-4636) | TTY: 1-888-232-6348 CDC/NIOSH INFO: cdc.gov/info | cdc.gov/niosh Monthly NIOSH eNews: https://www.cdc.gov/niosh/enews/ Suggested Citation NIOSH [2019]. Technical report: The NIOSH occupational exposure banding process for chemical risk management. By Lentz TJ, Seaton M, Rane P, Gilbert SJ, McKernan LT, Whit- taker C. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Dis- ease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2019-132, https://doi.org/10.26616/NIOSHPUB2019132.