Rapport En Français
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Campro Catalog Stable Isotope
Introduction & Welcome Dear Valued Customer, We are pleased to present to you our Stable Isotopes Catalog which contains more than three thousand (3000) high quality labeled compounds. You will find new additions that are beneficial for your research. Campro Scientific is proud to work together with Isotec, Inc. for the distribution and marketing of their stable isotopes. We have been working with Isotec for more than twenty years and know that their products meet the highest standard. Campro Scientific was founded in 1981 and we provide services to some of the most prestigious universities, research institutes and laboratories throughout Europe. We are a research-oriented company specialized in supporting the requirements of the scientific community. We are the exclusive distributor of some of the world’s leading producers of research chemicals, radioisotopes, stable isotopes and environmental standards. We understand the requirements of our customers, and work every day to fulfill them. In working with us you are guaranteed to receive: - Excellent customer service - High quality products - Dependable service - Efficient distribution The highly educated staff at Campro’s headquarters and sales office is ready to assist you with your questions and product requirements. Feel free to call us at any time. Sincerely, Dr. Ahmad Rajabi General Manager 180/280 = unlabeled 185/285 = 15N labeled 181/281 = double labeled (13C+15N, 13C+D, 15N+18O etc.) 186/286 = 12C labeled 182/282 = d labeled 187/287 = 17O labeled 183/283 = 13C labeleld 188/288 = 18O labeled 184/284 = 16O labeled, 14N labeled 189/289 = Noble Gases Table of Contents Ordering Information.................................................................................................. page 4 - 5 Packaging Information .............................................................................................. -

European Journal of Chemistry 5 (4) (2014) 681‐694
European Journal of Chemistry 5 (4) (2014) 681‐694 European Journal of Chemistry Journal homepage: www.eurjchem.com Synthesis, reactions and applications of pyranotriazolopyrimidines Ashraf Hassan Fekry Abd El‐Wahab a,b,*, Ibrahim Ali Radini a and Hany Mostafa Mohamed a,b a Chemistry Department, Faculty of Science, Jazan University, 2097, Jazan, Saudi Arabia b Chemistry Department, Faculty of Science, Al‐Azhar University, 11884, Nasr City, Cairo, Egypt *Corresponding author at: Chemistry Department, Faculty of Science, Jazan University, 2097, Jazan, Saudi Arabia. Tel.: +966.054.0963753. Fax: +966.017.3230028. E‐mail address: [email protected] (A.H.F.A. El‐Wahab). REVIEW INFORMATION ABSTRACT This review deals with synthesis, reactions and their applications of pyranotriazolo‐ pyrimidines. The main purpose of this review is present a survey of literatures on the reactivity of amino imino derivatives and carboxylic acid derivatives. Some of these reactions have been applied successfully to the synthesis of biological important compounds. DOI: 10.5155/eurjchem.5.4.681‐694.1087 Received: 30 April 2014 Received in revised form: 27 May 2014 Accepted: 27 May 2014 Online: 31 December 2014 KEYWORDS Naphthols Pyrimidine Biological activity Pyranopyrimidines α‐Cyanocinnamonitriles Carboxylic acid derivatives 1. Introduction chemical and biological view points, due to their diverse pharmacological activities, such as antitumor potency [19,20], Pyran derivatives have attracted a great deal of interest inhibition of KDR kinase [21], antifungal effect [22] and owing to their antimicrobial activity [1‐7], inhibition of influ‐ macrophage activation [23]. enza, virus sialidase [8], mutagenic activity [9], activity as antiviral [10], anti‐proliferaction agents [11], sex pheromones [12], antitumor [13] and anti‐inflammatory agents [14]. -

Fullerene-Acene Chemistry
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2007 Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene Andreas John Athans University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Athans, Andreas John, "Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene" (2007). Doctoral Dissertations. 363. https://scholars.unh.edu/dissertation/363 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. FULLERENE-ACENE CHEMISTRY: PART I: STUDIES ON THE REGIOSELECTIVE REDUCTION OF ACENES AND ACENE QUINONES; PART II: PROGRESS TOWARD THE SYNTHESIS OF LARGE ACENES AND THEIR DIELS- ALDER CHEMISTRY WITH [60]FULLERENE VOLUME 1 CHAPTERS 1-5 BY ANDREAS JOHN ATHANS B.S. University of New Hampshire, 2001 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy m Chemistry May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 3 2 6 0 5 8 6 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

Download Article (PDF)
SYNTHESIS OF FUSED AND NONFUSED HETEROCYCLES FROM 5- AND 8-SUBSTITUTED 3-HYDRAZINO-l,2,4-TRIAZINO[5,6-Z>]INDOLE Hamida Abdel Hamid, Ahmed Mousaad, El Sayed Ramadan and El Sayed H. El Ashry* Chemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt Abstract: The 7-and 10-substituted 1,2,4-triazolo[4\3':2,3][l,2,4]triazino[5,6-i]indoles were prepared by the reaction of 5- and 8-substituted-3-hydrazino-l,2,4-triazino[5,6-i>]indole with formic acid ortriethyl orthoformate. Reaction of the hydrazines with acetylacetone, ethyl acetoacetate and ethyl cyanoacetate gave the respective biheterocycles containing pvrazole and pyrazolone rings. Reaction of the hydrazines with carbondisulphide, thiourea, urea, ethyl chloroformate as well as nitrous acid have been investigated. Reaction of 5-allyl-3-methylthio-1,2,4-triazino[5,6-6]indole with ethanolamine followed by cyclization gave the imidazotriazinoindole. INTRODUCTION The l,2,4-triazino[5,6-i]indole ring has been found to be a useful carrier for various functional groups for developing antiviral and antibacterial activities [1-8], The imidazo, pyrimido or triazolo-1,2,4- triazino[5,6-6]indoles showed also antiviral and antibacterial properties [9], The arylidene moiety of 3- arylidene and heterocyclic formylidenehydrazono-l,2,4-triazino[5,6-&]indole derivatives has been found to play a role on their activity against Staphylococcus aureus and Bacillus cereus as well as Ρ 388 lymphocytic leukemia [8]. The 3-hydrazino-1,2,4-triazino[5,6-A]indole and 3-hydrazino-5,6-diphenyl-l,2,4-triazine have an effect on the carbohydrate metabolism and lipid components in rat [10,11], The regioselective annelation of a triazole ring to the 1,2,4-triazino[5,6-b] indole either via the dehydrative cyclization of the respective hydrazide or the dehydrogenative cyclization of the hydrazones has been reported [12-16]. -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -
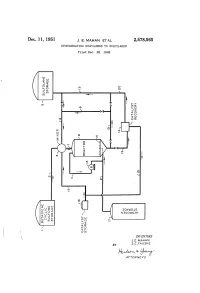
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0004.002 A1 Maywald Et Al
US 2011 00040O2A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0004.002 A1 Maywald et al. (43) Pub. Date: Jan. 6, 2011 (54) PROCESS FOR PREPARING ALKYL 2-ALKOXYMETHYLENE-44-DIFLUORO-3- OXOBUTYRATES O O (I) (75) Inventors: Volker Maywald, Ludwigshafen F (DE); Sebastian Peer Smidt, OR, Offersheim (DE); Bernd Wolf, F Fussgoenheim (DE); Christopher Koradin, Ludwigshafen (DE); b Thomas Zierke, Boehl-Iggelheim Rreacting (DE); Michael Rack, Eppelheim (DE); Michael Keil, Freinsheim (DE) (II) O Correspondence Address: ls BRINKS, HOFER, GILSON & LIONE HC OR, P.O. BOX 110285 alkyl acetate RESEARCH TRIANGLE PARK, NC 27709 (US) (III) ROM (73) Assignee: BASF SE, Ludwigshafen (DE) alkoxide, (21) Appl. No.: 12/919,842 where M is a sodium or potassium ion, and (22) PCT Filed: Feb. 27, 2009 (IV) (86). PCT No.: PCT/EP09/52378 S371 (c)(1), OR, (2), (4) Date: Aug. 27, 2010 F alkyl (30) Foreign Application Priority Data difluoroacetate Feb. 29, 2008 (EP) .................................. O81021974 without additional solvent to form an enolate (V) Publication Classification (V) OM O (51) Int. Cl. F N CO7D 231/4 (2006.01) OR, CD7C 69/66 (2006.01) (52) U.S. Cl. ...................................... 548/374.1; 560/177 b) releasing the corresponding alkyl 4,4-difluoroacetoacetate (I) from the enolate (V) by means of acid, c) removing the salt formed from cation Mandacid anion as (57) ABSTRACT a solid and d) converting (I), without isolation from the crude reaction A process for preparing alkyl 2-alkoxymethylene-4,4-dif mixture, to the alkyl 2-alkoxymethylene-4,4-difluoro-3- luoro-3-oxobutyrates (VI) oxobutyrate (VI), and the use of (VI) for preparing 1-methyl-3-difluoromethyl pyrazol-3-ylcarboxyates VII (VI) (VII) OR, FHC COOR. -

Hazardous Substances (Chemicals) Transfer Notice 2006
16551655 OF THURSDAY, 22 JUNE 2006 WELLINGTON: WEDNESDAY, 28 JUNE 2006 — ISSUE NO. 72 ENVIRONMENTAL RISK MANAGEMENT AUTHORITY HAZARDOUS SUBSTANCES (CHEMICALS) TRANSFER NOTICE 2006 PURSUANT TO THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 1656 NEW ZEALAND GAZETTE, No. 72 28 JUNE 2006 Hazardous Substances and New Organisms Act 1996 Hazardous Substances (Chemicals) Transfer Notice 2006 Pursuant to section 160A of the Hazardous Substances and New Organisms Act 1996 (in this notice referred to as the Act), the Environmental Risk Management Authority gives the following notice. Contents 1 Title 2 Commencement 3 Interpretation 4 Deemed assessment and approval 5 Deemed hazard classification 6 Application of controls and changes to controls 7 Other obligations and restrictions 8 Exposure limits Schedule 1 List of substances to be transferred Schedule 2 Changes to controls Schedule 3 New controls Schedule 4 Transitional controls ______________________________ 1 Title This notice is the Hazardous Substances (Chemicals) Transfer Notice 2006. 2 Commencement This notice comes into force on 1 July 2006. 3 Interpretation In this notice, unless the context otherwise requires,— (a) words and phrases have the meanings given to them in the Act and in regulations made under the Act; and (b) the following words and phrases have the following meanings: 28 JUNE 2006 NEW ZEALAND GAZETTE, No. 72 1657 manufacture has the meaning given to it in the Act, and for the avoidance of doubt includes formulation of other hazardous substances pesticide includes but -

OZO-L-CYCLOPBNTBNE. 1-CAEBOXYLATE and ETHYL 2-METHYL-3-OXOCYCLOPENTANECAKBOXYLATE
PART I THE SYNTHESES AND EEACTIONS OF METHYL 2-METHYL-^-OZO-l-CYCLOPBNTBNE. 1-CAEBOXYLATE AND ETHYL 2-METHYL-3-OXOCYCLOPENTANECAKBOXYLATE PAET II A STUDY OF THE REACTIONS OF GRIGNARD REAGENTS WITH ESTERS OF LEVULINIC ACID DISSERTATION Presented. In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By James Li McPherson, B.S., M.A, The Ohio State University 1955 Approved by: Adviser ACKNOWLEnXSMEWT It is a pleasure to express ray deep appreciation to Dr. Melvin S. Nevraan who suggested this problem, and whose advice and encouragement were an invaluable aid throughout this investigation, and to extend to the Upjohn Company, Kalamazoo, Michigan, ray sincere appreciation for a fellowship in connection with this work. il TABLE OF CONTENTS Aoknowledgeinent Part I The Syntheses and Beaotlons of Methyl S-raethyl-l-oxo-l-cyclopentene- 1-carhoxylate (IX) and ethyl 2-methyl~5-oxocyclopentanecarboxylate (Vl) Chapter Page I Introduction 1 II Synthesis 1. Discussion of Results 12 2. Flor Sheet (Fig. 5) 15 5. Diethyl 2-cyanc«3-taethylhutanedioate (ll) l6 k . Diethyl 2-cyano-2.-(2-oyanoethyl)-3- methylhutanedioate (ill) l8 5, Tfiethyl ^-metbyl-l, $,4-pentanetricarhoxylate (l?) 19 6 , Diethyl 2-methyl-3"Oxo«l,it— cyclopentane dicarhoxylate (v) 22 7, Ethyl 2~methyl-3-oxocyclopentanecar'boxylate (Vl) 2 k 8 , 2«Methyl*-3-'Oxo"l-cyclopentene-*l« carhoxylic acid (VIIl) 26 9* 2r^ethyl-3»-oxocyclopentaneoarh0xylic acid 29 10 0 Methyl 2p-methyl"3~oxo«l-cyclopentene" 1-carhoxylate (ix) 52 11. Ethyl 2-methyl~3'*oxor«l-cyclopentene" Imcarhoxylate (VIl) 55 III Reactions 1. -

B REGULATION (EC) No 1334/2008 of the EUROPEAN
2008R1334 — EN — 13.05.2016 — 011.001 — 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B REGULATION (EC) No 1334/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC (Text with EEA relevance) (OJ L 354, 31.12.2008, p. 34) Amended by: Official Journal No page date ►M1 Commission Implementing Regulation (EU) No 872/2012 of 1 October L 267 1 2.10.2012 2012 ►M2 Commission Regulation (EU) No 545/2013 of 14 June 2013 L 163 15 15.6.2013 ►M3 Commission Regulation (EU) No 985/2013 of 14 October 2013 L 273 18 15.10.2013 ►M4 Commission Regulation (EU) No 246/2014 of 13 March 2014 L 74 58 14.3.2014 ►M5 Commission Regulation (EU) No 1098/2014 of 17 October 2014 L 300 41 18.10.2014 ►M6 Commission Regulation (EU) 2015/648 of 24 April 2015 L 107 15 25.4.2015 ►M7 Commission Regulation (EU) 2015/1102 of 8 July 2015 L 181 54 9.7.2015 ►M8 Commission Regulation (EU) 2015/1760 of 1 October 2015 L 257 27 2.10.2015 ►M9 Commission Regulation (EU) 2016/54 of 19 January 2016 L 13 40 20.1.2016 ►M10 Commission Regulation (EU) 2016/55 of 19 January 2016 L 13 43 20.1.2016 ►M11 Commission Regulation (EU) 2016/178 of 10 February 2016 L 35 6 11.2.2016 ►M12 Commission Regulation (EU) 2016/637 of 22 April 2016 L 108 24 23.4.2016 2008R1334 — EN — 13.05.2016 — 011.001 -

United States Patent Office Patented Nov
3,215,732 United States Patent Office Patented Nov. 2, 1965 1. 2 example isopropanol. The reduction product may be 3,215,732 reacted with the said amine in the presence of a diluent NAPHTHALENE DERVATIVES or solvent for example ethanol and the process may be John S. Stephenson, Taplow, England, assignor to Im accelerated or completed by the application of heat. perial Chemical industries Limited, London, England, It is to be understood that the said reduction product a corporation of Great Britain No Drawing. Fied Apr. 26, 1961, Ser. No. 105,571. is believed to be one or other of the two naphthalene Claims priority, application Great Britain, May 4, 1960, derivatives of the formula: 15,716/60; Oct. 7, 1960, 34,438/60 CHOICH2X 9 Caims. (C. 260-501) 10 This invention relates to organic compounds and more Y particularly it relates to new naphthalene derivatives which possess valuable therapeutic properties. According to the invention I provide naphthalene and 15 derivatives of the formula: O CHOECEINR 1Ri of SCH, Y Y 20 NX, Z. wherein R stands for hydrogen or for a metay1 lauva. wherein R1 stands for hydrogen or for a methyl radical, R2 stands for a branched chain alkyl radical of not a mixture thereof, and either of these compounds or a more than 4 carbon atoms and Y and Z stand for hy mixture thereof can be used as starting material for the drogen, halogen, lower alkyl and lower alkoxy, and the 25 manufacture of the compounds of the present invention. non-toxic, pharmaceutically-acceptable salts thereof.