Lifetimes of Deeply Embedded Positrons in Metals Wallace William Souder Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stable Isotopes of Cobalt Properties of Cobalt
Stable Isotopes of Cobalt Isotope Z(p) N(n) Atomic Mass Natural Abundance Nuclear Spin Co-59 27 32 58.93320 100.00% 7/2- Cobalt was discovered in 1735 by Georg Brandt. Its name derives from the German word kobald, meaning "goblin" or "evil spirit." Minerals containing cobalt were used by the early civilizations of Egypt and Mesopotamia for coloring glass deep blue. Cobalt oxide is used today to add a pink or blue color to glass. It is also an important trace element in soils and necessary for animal nutrition. The most important modern use of cobalt is in the manufacture of various wear-resistant and superalloys. Its alloys have shown high resistance to corrosion and oxidation at high temperatures. Radioactive Cobalt-60 is used in radiography and in the sterilization of food. A silvery-white, shining, hard, ductile, somewhat malleable metal, cobalt is also ferromagnetic, with permeability two-thirds that of iron. It has exceptional magnetic properties in alloys. It is attached by dilute hydrochloric and sulfuric acids. It corrodes readily in air, and it has unusual coordinating properties, especially the trivalent ion. It is noncombustible except in powder form. Cobalt occurs in two allotropic modifications over a wide range of temperatures: the crystalline close-packed- hexagonal form is known as the alpha form, which turns into the beta (or gamma) form above 417 ºC. In finely powdered form, cobalt ignites spontaneously in air. Reactions with acetylene and bromine pentafluoride proceed to incandescence and can become violent. The metal is moderately toxic by ingestion. Inhalation of dusts can damage lungs. -

High Accuracy Measurement of Isotope Ratios of Molybdenum in Some Terrestrial Molybdenites
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ARTICLES High Accuracy Measurement of Isotope Ratios of Molybdenum in Some Terrestrial Molybdenites Qi-Lu* and Akimasa Masuda Department of Chemistry, Faculty of Science, The University of Tokyo, Tokyo, Japan The isotope ratios of molybdenum in molybdenites were studied. A special triple filament technique was used to obtain stable and lasting signals for MO+. There are no differences bigger than ~0.4 parts per IO4 among four samples and the standard. CJ Am Sot Mass Spectrom 1992, 3, IO- 17) olybdenum is a very interesting element be- denum thus far, in spite of the potential importance cause its seven isotopes can reflect several of research in isotopic abundance of molybdenum. M effects related to nuclear physics. The nu- In this study we have established a method for clear phenomena that may affect the isotope ratios in securing stable and lasting current of MO+ and exam- question are (1) the synthesis of seven isotopes of MO ined the mass fractionation of MO isotopes during involving three processes (r, s, and p) in the standard measurement. Based on these studies, the isotope model of nucleosynthesis [I]; ($2 the nuclear hssion of ratios of MO were determined with high precision uranium-238, which produces MO, “MO, 98Mo, and for some molybdenites from a variety of locations ‘“MO; and (3) the double-beta decays of ‘“Zr and throughout the world. The present study will afford a lwMo leading to 96Mo and ‘“Ru. Another intriguing foundation for further precise studies of molybdenum property of this element is that the anomalous abun- isotopes involving meteorites and terrestrial rocks. -

Isotope Production Potential at Sandia National Laboratories: Product, Waste, Packaging, and Transportation*
Isotope Production Potential at Sandia National Laboratories: Product, Waste, Packaging, and Transportation* A. J. Trennel Transportation Systems Department *- *-, o / /"-~~> Sandia National Laboratories** ' J Albuquerque, NM 87185 O Q T » Abstract The U.S. Congress directed the U.S. Department of Energy to establish a domestic source of molybdenum-99, an essential isotope used in nuclear medicine and radiopharmacology. An Environmental Impact Statement for production of 99Mo at one of four candidate sites is being prepared. As one of the candidate sites, Sandia National Laboratories is developing the Isotope Production Project. Using federally approved processes and procedures now owned by the U.S. Department of Energy, and existing facilities that would be modified to meet the production requirements, the Sandia National Laboratories' Isotope Project would manufacture up to 30 percent of the U.S. market, with the capacity to meet 100 percent of the domestic need if necessary. This paper provides a brief overview of the facility, equipment, and processes required to produce isotopes. Packaging and transportation issues affecting both product and waste are addressed, and the storage and disposal of the four low-level radioactive waste types generated by the production program are considered. Recommendations for future development are provided. This work was performed at Sandia National Laboratories, Albuquerque, New Mexico, for the U.S. Department of Energy under Contract DE-AC04-94AL85000. A U.S. Department of Energy facility. DISTRPJTO OF THIS DOCUMENT IS UNLIMITED #t/f W A8 1 fcll PROJECT NEED AND BACKGROUND Nuclear medicine is an expanding segment of today's medical and pharmaceutical communities. Specific radioactive isotopes are vital, with molybdenum-99 (99Mo) being the most important medical isotope. -

Annual Report 1951 National Bureau of Standards
Annual Report 1951 National Bureau of Standards Miscellaneous Publication 204 UNITED STATES DEPARTMENT OF COMMERCE Charles Sawyer, Secretary NATIONAL BUREAU OF STANDARDS A. V. Astint, Director Annual Report 1951 National Bureau of Standards For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 2 5, D. C. Price 50 cents CONTENTS Page 1. General Review 1 2. Electricity 16 Beam intensification in a high-voltage oscillograph 17, Low-temperature dry cells 17, High-rate batteries 17, Battery additives 18. 3. Optics and Metrology 18 The kinorama 19, Measurement of visibility for aircraft 20, Antisubmarine aircraft searchlights 20, Resolving power chart 20, Refractivity 21, Thermal expansivity of aluminum alloys 21. 4. Heat and Power . 21 Thermodynamic properties of materials 22, Synthetic rubber and other high polymers 23, Combustors for jet engines 24, Temperature and composition of flames 25, Engine "knock" 25, Low-temperature physics 26, Medical physics instrumentation 28. 5. Atomic and Radiation Physics 28 Atomic standard of length 29, Magnetic moment of the proton 29, Spectra of artificial elements 31, Photoconductivity of semiconductors 31, Radiation detecting instruments 32, Protection against radiation 32, X-ray equipment 33, Atomic and molecular ions 35, Electron physics 35, Tables of nuclear data 35, Atomic energy levels 36. 6. Chemistry 36 Radioactive carbohydrates 36, Dextran as a substitute for blood plasma 37, Acidity and basicity in organic solvents 37, Interchangeability of fuel gases 38, Los Angeles "smog" 39, Infrared spectra of alcohols 39, Electrodeposi- tion 39, Development of analytical methods 40, Physical constants 42. 7. Mechanics 42 Turbulent flow 43, Turbulence at supersonic speeds 43, Dynamic properties of materials 43, High-frequency vibrations 44, Hearing loss 44, Physical properties by sonic methods 44, Water waves 46, Density currents 46, Precision weighing 46, Viscosity of gases 46, Evaporated thin films 47. -

How Earth Got Its Moon Article
FEATURE How Earth Got its MOON Standard formation tale may need a rewrite By Thomas Sumner he moon’s origin story does not add up. Most “Multiple impacts just make more sense,” says planetary scientists think that the moon formed in the earli- scientist Raluca Rufu of the Weizmann Institute of Science in est days of the solar system, around 4.5 billion years Rehovot, Israel. “You don’t need this one special impactor to Tago, when a Mars-sized protoplanet called Theia form the moon.” whacked into the young Earth. The collision sent But Theia shouldn’t be left on the cutting room floor just debris from both worlds hurling into orbit, where the rubble yet. Earth and Theia were built largely from the same kind of eventually mingled and combined to form the moon. material, new research suggests, and so had similar composi- If that happened, scientists expect that Theia’s contribution tions. There is no sign of “other” material on the moon, this would give the moon a different composition from Earth’s. perspective holds, because nothing about Theia was different. Yet studies of lunar rocks show that Earth and its moon are “I’m absolutely on the fence between these two opposing compositionally identical. That fact throws a wrench into the ideas,” says UCLA cosmochemist Edward Young. Determining planet-on-planet impact narrative. which story is correct is going to take more research. But the Researchers have been exploring other scenarios. Maybe answer will offer profound insights into the evolution of the the Theia impact never happened (there’s no direct evidence early solar system, Young says. -
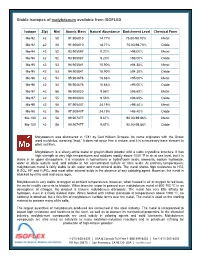
Stable Isotopes of Molybdenum Available from ISOFLEX
Stable isotopes of molybdenum available from ISOFLEX Isotope Z(p) N(n) Atomic Mass Natural Abundance Enrichment Level Chemical Form Mo-92 42 50 91.906810 14.77% 75.00-98.70% Metal Mo-92 42 50 91.906810 14.77% 75.00-98.70% Oxide Mo-94 42 52 93.905087 9.23% >98.00% Metal Mo-94 42 52 93.905087 9.23% >98.00% Oxide Mo-95 42 53 94.905841 15.90% ≥94.30% Metal Mo-95 42 53 94.905841 15.90% ≥94.30% Oxide Mo-96 42 54 95.904678 16.68% >95.00% Metal Mo-96 42 54 95.904678 16.68% >95.00% Oxide Mo-97 42 55 96.906020 9.56% ≥96.60% Metal Mo-97 42 55 96.906020 9.56% ≥96.60% Oxide Mo-98 42 56 97.905407 24.19% >98.40% Metal Mo-98 42 56 97.905407 24.19% >98.40% Oxide Mo-100 42 58 99.907477 9.67% 90.00-99.86% Metal Mo-100 42 58 99.907477 9.67% 90.00-99.86% Oxide Molybdenum was discovered in 1781 by Carl William Scheele. Its name originates with the Greek word molybdos, meaning “lead.” It does not occur free in nature, and it is a necessary trace element in plant nutrition. Molybdenum is a silvery-white metal or grayish-black powder with a cubic crystalline structure. It has high strength at very high temperatures and oxidizes rapidly above 1000 °F in air at sea level, but it is stable in an upper atmosphere. -

Optimization Studies of the CERN-ISOLDE Neutron Converter and Fission Target System
Optimization Studies of the CERN-ISOLDE neutron converter and fission target system Raul Luís1, José G. Marques, Thierry Stora, Pedro Vaz, Luca Zanini 1ITN – Estrada Nacional 10, 2686-953, Sacavém, Portugal 2CERN – CH-1211, Genève 23, Switzerland 3PSI – 5232 Villigen, Switzerland E-mail: [email protected] Abstract. The ISOLDE facility at CERN has been one of the leading isotope separator on-line (ISOL) facilities worldwide since it became operative in 1967. More than 1000 isotopes are available at ISOLDE, produced after the bombardment of various primary targets with a pulsed proton beam of energy 1.4 GeV and an average intensity of 2 μA. A tungsten solid neutron converter has been used for ten years to produce neutron-rich fission fragments in UCx targets. In this work, the Monte Carlo code FLUKA and the cross section codes TALYS and ABRABLA were used to study the current layout of the neutron converter and fission target system of the ISOLDE facility. An optimized target system layout is proposed, which maximizes the production of neutron-rich isotopes and reduces the contamination by undesired proton-rich isobars. The studies here reported can already be applied to ISOLDE and will be of special relevance for the future facilities HIE-ISOLDE and EURISOL. 1. Introduction One of the most efficient ways to study nuclei far from stability is the Isotope Separation On-Line (ISOL) method, in which a beam of high-energy hadrons hits a thick target, producing a great variety of radioactive products through different processes like fission, spallation and fragmentation [1]. The resulting nuclides can be studied after they are extracted from the target, ionized and mass separated. -

PSI • Scientific Report 1999 /Volume I
CH0000002 PAUL SCHERRER INSTITUT ISSN 1423-7296 March 2000 PSI • Scientific Report 1999 /Volume I Particles and Matter 3 1/28 An international collaboration of radiochemists led by the Laboratory for Radiochemistry and Environmental Chemistry of PSl and Bern University succeeded for the first time to experimentally investigate the chemical properties of bohrium (element 107) and to establish it as a member of group 7 of the Periodic Table. During a one-month long experiment, a total of only six bohrium atoms were gaschromatographically isolated in the form of volatile bohrium oxychloride molecules and identified by registering their unique decay sequence of alpha particle emissions via dubnium (element 105) to lawrencium (element 103). The short-lived bohrium atoms, decaying with a half-life of about 20 s, were produced by bombarding a very rare, highly radioactive berkelium target supplied by the US department of energy with an intense beam of neon ions at the PSl injector 1 cyclotron. PAUL SCHERRER INSTITUT ISSN 1423-7296 March 2000 \} Scientific Report 1999 Volume I Particles and Matter ed. by: J. Gobrecht, H. Gaggeler, D. Herlach, K. Junker, P.-R. Kettle, P. Kubik, A. Zehnder CH-5232 Villigen PSI Switzerland Telephone:+41 56 310 21 11 Telefax:+ 41 56 310 21 99 http://www.psi.ch TABLE OF CONTENTS Introduction .1 Laboratory for Particle Physics 3 Foreword 4 Particle Physics Theory Theory (I) 5 Theory (II) 6 Ring Accelerator Experiments Particle Properties and Decays A precise measurement of the Jt+-^7t°e+v decay rate 7 Measurement of -

Chapter 2 Atoms, Molecules and Ions
Chapter 2 Atoms, Molecules and Ions PRACTICING SKILLS Atoms:Their Composition and Structure 1. Fundamental Particles Protons Electrons Neutrons Electrical Charges +1 -1 0 Present in nucleus Yes No Yes Least Massive 1.007 u 0.00055 u 1.007 u 3. Isotopic symbol for: 27 (a) Mg (at. no. 12) with 15 neutrons : 27 12 Mg 48 (b) Ti (at. no. 22) with 26 neutrons : 48 22 Ti 62 (c) Zn (at. no. 30) with 32 neutrons : 62 30 Zn The mass number represents the SUM of the protons + neutrons in the nucleus of an atom. The atomic number represents the # of protons, so (atomic no. + # neutrons)=mass number 5. substance protons neutrons electrons (a) magnesium-24 12 12 12 (b) tin-119 50 69 50 (c) thorium-232 90 142 90 (d) carbon-13 6 7 6 (e) copper-63 29 34 29 (f) bismuth-205 83 122 83 Note that the number of protons and electrons are equal for any neutral atom. The number of protons is always equal to the atomic number. The mass number equals the sum of the numbers of protons and neutrons. Isotopes 7. Isotopes of cobalt (atomic number 27) with 30, 31, and 33 neutrons: 57 58 60 would have symbols of 27 Co , 27 Co , and 27 Co respectively. Chapter 2 Atoms, Molecules and Ions Isotope Abundance and Atomic Mass 9. Thallium has two stable isotopes 203 Tl and 205 Tl. The more abundant isotope is:___?___ The atomic weight of thallium is 204.4 u. The fact that this weight is closer to 205 than 203 indicates that the 205 isotope is the more abundant isotope. -

Molybdenum in the Open Cluster Stars
MOLYBDENUM IN THE OPEN CLUSTER STARS T. Mishenina1, E. Shereta1, M. Pignatari2,3,6,7, G. Carraro4, T. Gorbaneva1, C. Soubiran5 1Astronomical Observatory, Odessa National University, Marazlievskaia Str., 1V, 65014, Odessa, Ukraine [email protected] 2 E.A. Milne Centre for Astrophysics, Department of Physics & Mathematics, University of Hull, HU6 7RX, United Kingdom [email protected] 3 Konkoly Observatory, Hungarian Academy of Sciences, Konkoly Thege Miklos ut 15-17, H-1121 Budapest, Hungary 4 Dipartimento di Fisica e Astronomia, Universita di Padova, I-35122, Padova, Italy [email protected] 5 Laboratoire d’astrophysique de Bordeaux, Universit´eBordeaux, CNRS, B18N, all´ee Geoffroy Saint-Hilaire, 33615 Pessac, France [email protected] 6 Joint Institute for Nuclear Astrophysics - Center for the Evolution of the Elements, USA 7 NuGrid Collaboration https://nugrid.github.io/ June 9, 2020 arXiv:2006.04629v1 [astro-ph.GA] 8 Jun 2020 Abstract Molybdenum abundances in the stars from 13 different open clusters were determined. High-resolution stellar spectra were obtained using the VLT tele- scope equipped with the UVES spectrograph on Cerro Paranal, Chile. The Mo abundances were derived in the LTE approximation from the Mo I lines at 5506 A˚ and 5533 A.˚ A comparative analysis of the behaviour of molyb- denum in the sampled stars of open clusters and Galactic disc show similar trends of decreasing Mo abundances with increasing metallicities; such a be- haviour pattern suggests a common origin of the examined populations. On the other hand, the scatter of Mo abundances in the open cluster stars is slightly greater, 0.14 dex versus 0.11 dex. -

Concurrent Reduction and Distillation; an Improved Technique for the Recovery and Chemical Refinement of the Isotopes of Cadmium and Zinc*
If I CONCURRENT REDUCTION AND DISTILLATION; AN IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC* H. H. Cardill L. E. McBride LOHF-821046—2 E. W. McDaniel DE83 001931 Operations Division Oak Ridge National Laboratory Oak Ridge, Tennessee 3 7830 .DISCLAIMER • s prepared es an accouni oi WOTk sconsQ'ed by an agency of itie United Slates Cover r "iiit?3 Stilt 65 Gov(?fnft»gn ^ ri(jt ^nv aQcncy Tllf GO* ^or rlny O^ tt^^ir ^rnployut^. TigV MASTER lostifl. ion, apod'aiuj pioiJut;. <y vned nghtv Reference hcein xc any v>t^-'T*t. ^ Dv ^.r^de narno, tf^Oernafk^ mir'ij'iJClureT. or otrierw «d <Jo*^ •nenaalion. or liivONng bv 'Vhe United _^_ . vs and opinions of authors enp'«i«] herein do noi IOM t' irip UnitK* Stales Govefnrneni c a^v agency For presentation at the 11th World Conference of the International Nuclear Target Development Society, October 6-8, 1982, Seattle, Washington. By acceptance of this article, the publisher or recipient acknowledge* the U.S. Govarnment's right to retain a nonaxclusiva, royalty-frtta license in and to any copyright covering tha article. NOTICE P0T09_0£-Jfns,J^SPQRT_JfgE_Il,LESIBLE. I has been reproauce.1 from the best available aopy to pensit the broadest possible avail- ability* *Research sponsored by the Office of Basic Energy Science, U.S. Department of Energy under contract W-7405-eng-26 with the Union Carbide Co rpcration,, DISTRIBUTION OF THIS DOCUMEHT 18 UNLIMITED ' CONCURRENT REDUCTION AND DISTILLATION - AY. IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC H. -

PRODUCTION of Mo-99/Tc-99M VIA PHOTONEUTRON REACTION USING
PRODUCTION OF Mo-99/Tc-99m VIA PHOTONEUTRON REACTION USING NATURAL MOLYBDENUM A Thesis by TALAL BADER HARAHSHEH Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Chair of Committee, Gamal Akabani Co-Chair of Committee, John W. Poston, Sr. Committee Member, Victor M. Ugaz Head of Department, Yassin A. Hassan May 2017 Major Subject: Nuclear Engineering Copyright 2017 Talal Harahsheh ABSTRACT The current shortage of the widely used radionuclide 99mTc has raised many concerns in the nuclear medicine industry. The shortage is caused by the outdated method of production using highly enriched uranium (HEU) and nuclear reactors. This production method is no longer feasible due to the restrictions on the use of HEU. Alternative methods of production must be addressed to ensure the uninterrupted supply of this key radionuclide. This research analyzes an alternative production method of 99mTc using an accelerator-generated 99Mo, which is the parent element of 99mTc. The radionuclide 99mTc has a short half-life of 6 hours. However, 99Mo, the parent nuclide has a much longer half-life of 66 hours. Usually, a medical facility receives a 99Mo/99mTc generator. Technetium-99m would be obtained from the 99Mo provided through chemical separation, which is conducted at the medical facilities. The main issues to be addressed include the production of 99Mo using the photonuclear reaction, the containment and exclusion of unwanted decay products, ensuring the integrity of the end-product 99Mo/99mTc post chemical separation, and assessing the economic feasibility of the complete strategy, including life cycle.