Stable Isotope Separation in Calutrons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Strontium-90
EPA Facts about Strontium-90 What is strontium-90? The most common isotope of strontium is strontium-90. Radioactive strontium-90 is produced when uranium and plutonium undergo fission. Fission The time required for a radioactive substance to is the process in which the nucleus of a lose 50 percent of its radioactivity by decay is radionuclide breaks into smaller parts. Large known as the half-life. Strontium-90 has a half- amounts of radioactive strontium-90 were life of 29 years and emits beta particles of produced during atmospheric nuclear weapons relatively low energy as it decays. Yttrium-90, its tests conducted in the 1950s and 1960s. As a decay product, has a shorter half-life (64 hours) result of atmospheric testing and radioactive than strontium-90, but it emits beta particles of fallout, this strontium was dispersed and higher energy. deposited on the earth. How are people exposed to strontium- 90? What are the uses of strontium-90? Although external exposure to strontium-90 Strontium-90 is used in medical and agricultural from nuclear testing is of minor concern because studies. It is also used in thermoelectric devices environmental concentrations are low, that are built into small power supplies for use strontium in the environment can become part of the food chain. This pathway of exposure in remote locations, such as navigational beacons, remote weather stations, and space became a concern in the 1950s with the advent vehicles. Additionally, strontium-90 is used in of atmospheric testing of nuclear explosives. electron tubes, radioluminescent markers, as a With the suspension of atmospheric testing of radiation source in industrial thickness gauges, nuclear weapons, dietary intake has steadily and for treatment of eye diseases. -

Calcined Bone Provides a Reliable Substrate for Strontium Isotope Ratios As Shown by an Enrichment Experiment
Research Article Received: 8 August 2014 Revised: 15 October 2014 Accepted: 16 October 2014 Published online in Wiley Online Library Rapid Commun. Mass Spectrom. 2015, 29, 107–114 (wileyonlinelibrary.com) DOI: 10.1002/rcm.7078 Calcined bone provides a reliable substrate for strontium isotope ratios as shown by an enrichment experiment Christophe Snoeck1*, Julia Lee-Thorp1,RickSchulting1,JeroendeJong2, Wendy Debouge2 and Nadine Mattielli2 1Research Laboratory for Archaeology and the History of Art, University of Oxford, Dyson Perrins Building, South Parks Rd, Oxford OX1 3QY, UK 2G-Time Laboratory, Université Libre de Bruxelles, CP 160/02, 50, Avenue F.D. Roosevelt, B-1050 Brussels, Belgium RATIONALE: Strontium isotopes (87Sr/86Sr) are used in archaeological and forensic science as markers of residence or mobility because they reflect the local geological substrate. Currently, tooth enamel is considered to be the most reliable tissue, but it rarely survives heating so that in cremations only calcined bone fragments survive. We set out to test the proposition that calcined bone might prove resistant to diagenesis, given its relatively high crystallinity, as the ability to measure in vivo 87Sr/86Sr from calcined bone would greatly extend application to places and periods in which cremation was the dominant mortuary practice, or where unburned bone and enamel do not survive. METHODS: Tooth enamel and calcined bone samples were exposed to a 87Sr-spiked solution for up to 1 year. Samples were removed after various intervals, and attempts were made to remove the contamination using acetic acid washes and ultrasonication. 87Sr/86Sr was measured before and after pre-treatment on a Nu Plasma multi-collector induced coupled plasma mass spectrometer using NBS987 as a standard. -

Mass Independent Fractionation of Mercury Isotopes As Source Tracers in Sediments
Available online at www.sciencedirect.com ScienceDirect Procedia Earth and Planetary Science 13 ( 2015 ) 151 – 157 11th Applied Isotope Geochemistry Conference, AIG-11 BRGM Mass Independent Fractionation of Mercury Isotopes as Source Tracers in Sediments Reshmi Dasa,b,* , William Landingb, Michael Bizimisc, Leroy Odomb, Jane Caffreyd aEarth Observatory of Singapore, 50 Nanyang Avenue, Singapore 639798, Singapore bFlorida State University, Tallahassee, Florida 32306, USA cUniversity of South Carolina, Columbia, South Carolina 29208, USA dUniversity of West Florida, Pensacola, Florida 32514, USA Abstract Since the discovery of isotopic fractionation of mercury in nature, mass dependent fractionation (MDF) and mass independent fractionation (MIF) of mercury isotopes are used as a tracer to understand the mercury cycle. MIF is a powerful tool in understanding the Hg transformations and reaction mechanisms. Here we look into the MIF of the two odd isotopes of mercury (199Hg and 201Hg) in sediment samples collected from lakes and springs of Florida, Lake Erie, and Yucatan Peninsula. The '199Hg and '201Hg of the sediments range from + 0.52‰ to -0.48‰. From the isotopic signature we interpret the possible source of Hg in the Yucatan Peninsula carbonate to be Hg(II) from the water column. Hg in the Florida lakes and spring sediments primarily comes from litterfall. Lake Erie appears to have an anthropogenic source. This study suggests that the MIF signature of Hg isotopes can be used to qualitatively determine the primary source(s) of Hg in sediments. © 2015 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license © 2015 The Authors. -

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements . -
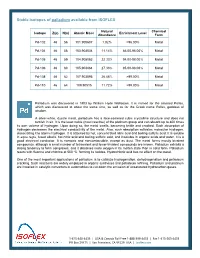
Stable Isotopes of Palladium Available from ISOFLEX
Stable isotopes of palladium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Pd-102 46 56 101.905607 1.02% >96.00% Metal Pd-104 46 58 103.904034 11.14% 86.00-98.00% Metal Pd-105 46 59 104.905083 22.33% 94.00-98.00% Metal Pd-106 46 60 105.903484 27.33% 95.00-98.00% Metal Pd-108 46 62 107.903895 26.46% >99.00% Metal Pd-110 46 64 109.90515 11.72% >99.00% Metal Palladium was discovered in 1803 by William Hyde Wollaston. It is named for the asteroid Pallas, which was discovered at about the same time, as well as for the Greek name Pallas, goddess of wisdom. A silver-white, ductile metal, palladium has a face-centered cubic crystalline structure and does not tarnish in air. It is the least noble (most reactive) of the platinum group and can absorb up to 800 times its own volume of hydrogen. Upon doing so, the metal swells, becoming brittle and cracked. Such absorption of hydrogen decreases the electrical conductivity of the metal. Also, such absorption activates molecular hydrogen, dissociating it to atomic hydrogen. It is attacked by hot, concentrated nitric acid and boiling sulfuric acid. It is soluble in aqua regia, fused alkalis, hot nitric acid and boiling sulfuric acid, and insoluble in organic acids and water. It is a good electrical conductor. It is nontoxic and noncombustible, except as dust. The metal forms mostly bivalent compounds, although a small number of tetravalent and fewer trivalent compounds are known. -

How Earth Got Its Moon Article
FEATURE How Earth Got its MOON Standard formation tale may need a rewrite By Thomas Sumner he moon’s origin story does not add up. Most “Multiple impacts just make more sense,” says planetary scientists think that the moon formed in the earli- scientist Raluca Rufu of the Weizmann Institute of Science in est days of the solar system, around 4.5 billion years Rehovot, Israel. “You don’t need this one special impactor to Tago, when a Mars-sized protoplanet called Theia form the moon.” whacked into the young Earth. The collision sent But Theia shouldn’t be left on the cutting room floor just debris from both worlds hurling into orbit, where the rubble yet. Earth and Theia were built largely from the same kind of eventually mingled and combined to form the moon. material, new research suggests, and so had similar composi- If that happened, scientists expect that Theia’s contribution tions. There is no sign of “other” material on the moon, this would give the moon a different composition from Earth’s. perspective holds, because nothing about Theia was different. Yet studies of lunar rocks show that Earth and its moon are “I’m absolutely on the fence between these two opposing compositionally identical. That fact throws a wrench into the ideas,” says UCLA cosmochemist Edward Young. Determining planet-on-planet impact narrative. which story is correct is going to take more research. But the Researchers have been exploring other scenarios. Maybe answer will offer profound insights into the evolution of the the Theia impact never happened (there’s no direct evidence early solar system, Young says. -

(12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden Et Al
USOO8625731B2 (12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden et al. (45) Date of Patent: Jan. 7, 2014 (54) COMPACT NEUTRONGENERATOR FOR (51) Int. Cl. MEDICAL AND COMMERCIAL SOTOPE G2G 4/02 (2006.01) PRODUCTION, FISSION PRODUCT G2G 4/00 (2006.01) PURIFICATION AND CONTROLLED (52) U.S. Cl. GAMMA REACTIONS FOR DIRECT USPC ............................ 376/108: 376/112:376/156 ELECTRIC POWER GENERATION (58) Field of Classification Search USPC .......................... 376/157, 108,156, 171, 172 (76) Inventors: Charles S. Holden, San Francisco, CA See application file for complete search history. (US); Robert E. Schenter, Portland, OR (US) (56) References Cited *) Notice: Subject to anyy disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 3,748,226 A * 7/1973 Ribe et al. ..................... 376,124 U.S.C. 154(b) by 961 days. 3,778,627 A * 12/1973 Carpenter ...... ... 376, 192 4,749,540 A * 6/1988 Bogartet al. .. ... 376/133 (21) Appl. No.: 12/296,844 4,997,619 A * 3/1991 Pettus ............ ... 376,288 2004/02284.33 A1* 1 1/2004 Magill et al. ... 376/347 (22) PCT Filed: Apr. 13, 2007 2005/022O248 A1* 10, 2005 Ritter ...... ... 376/190 2008/O144762 A1* 6/2008 Holden et al. ..... ... 376/416 (86). PCT No.: PCT/US2OOTAO66668 * cited by examiner S371 (c)(1), (2), (4) Date: Oct. 10, 2008 Primary Examiner — Jack W Keith Assistant Examiner — Sean P Burke (87) PCT Pub. No.: WO2008/060663 (74) Attorney, Agent, or Firm — Craig M. Stainbrook; PCT Pub. Date: May 22, 2008 Stainbrook & Stainbrook, LLP (65) Prior Publication Data (57) ABSTRACT A neutron generator and isotope production apparatus and US 2011 FO268237 A1 Nov. -

Optimization Studies of the CERN-ISOLDE Neutron Converter and Fission Target System
Optimization Studies of the CERN-ISOLDE neutron converter and fission target system Raul Luís1, José G. Marques, Thierry Stora, Pedro Vaz, Luca Zanini 1ITN – Estrada Nacional 10, 2686-953, Sacavém, Portugal 2CERN – CH-1211, Genève 23, Switzerland 3PSI – 5232 Villigen, Switzerland E-mail: [email protected] Abstract. The ISOLDE facility at CERN has been one of the leading isotope separator on-line (ISOL) facilities worldwide since it became operative in 1967. More than 1000 isotopes are available at ISOLDE, produced after the bombardment of various primary targets with a pulsed proton beam of energy 1.4 GeV and an average intensity of 2 μA. A tungsten solid neutron converter has been used for ten years to produce neutron-rich fission fragments in UCx targets. In this work, the Monte Carlo code FLUKA and the cross section codes TALYS and ABRABLA were used to study the current layout of the neutron converter and fission target system of the ISOLDE facility. An optimized target system layout is proposed, which maximizes the production of neutron-rich isotopes and reduces the contamination by undesired proton-rich isobars. The studies here reported can already be applied to ISOLDE and will be of special relevance for the future facilities HIE-ISOLDE and EURISOL. 1. Introduction One of the most efficient ways to study nuclei far from stability is the Isotope Separation On-Line (ISOL) method, in which a beam of high-energy hadrons hits a thick target, producing a great variety of radioactive products through different processes like fission, spallation and fragmentation [1]. The resulting nuclides can be studied after they are extracted from the target, ionized and mass separated. -

The Discoverers of the Ruthenium Isotopes
•Platinum Metals Rev., 2011, 55, (4), 251–262• The Discoverers of the Ruthenium Isotopes Updated information on the discoveries of the six platinum group metals to 2010 http://dx.doi.org/10.1595/147106711X592448 http://www.platinummetalsreview.com/ By John W. Arblaster This review looks at the discovery and the discoverers Wombourne, West Midlands, UK of the thirty-eight known ruthenium isotopes with mass numbers from 87 to 124 found between 1931 and 2010. Email: [email protected] This is the sixth and fi nal review on the circumstances surrounding the discoveries of the isotopes of the six platinum group elements. The fi rst review on platinum isotopes was published in this Journal in October 2000 (1), the second on iridium isotopes in October 2003 (2), the third on osmium isotopes in October 2004 (3), the fourth on palladium isotopes in April 2006 (4) and the fi fth on rhodium isotopes in April 2011 (5). An update on the new isotopes of palladium, osmium, iridium and platinum discovered since the previous reviews in this series is also included. Naturally Occurring Ruthenium Of the thirty-eight known isotopes of ruthenium, seven occur naturally with the authorised isotopic abun- dances (6) shown in Table I. The isotopes were fi rst detected in 1931 by Aston (7, 8) using a mass spectrograph at the Cavendish Laboratory, Cambridge University, UK. Because of diffi cult experimental conditions due to the use of poor quality samples, Aston actually only detected six of the isotopes and obtained very approximate Table I The Naturally Occurring Isotopes of Ruthenium Mass number Isotopic Abundance, % 96Ru 5.54 98Ru 1.87 99Ru 12.76 100Ru 12.60 101Ru 17.06 102Ru 31.55 104Ru 18.62 251 © 2011 Johnson Matthey http://dx.doi.org/10.1595/147106711X592448 •Platinum Metals Rev., 2011, 55, (4)• percentage abundances. -

Concurrent Reduction and Distillation; an Improved Technique for the Recovery and Chemical Refinement of the Isotopes of Cadmium and Zinc*
If I CONCURRENT REDUCTION AND DISTILLATION; AN IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC* H. H. Cardill L. E. McBride LOHF-821046—2 E. W. McDaniel DE83 001931 Operations Division Oak Ridge National Laboratory Oak Ridge, Tennessee 3 7830 .DISCLAIMER • s prepared es an accouni oi WOTk sconsQ'ed by an agency of itie United Slates Cover r "iiit?3 Stilt 65 Gov(?fnft»gn ^ ri(jt ^nv aQcncy Tllf GO* ^or rlny O^ tt^^ir ^rnployut^. TigV MASTER lostifl. ion, apod'aiuj pioiJut;. <y vned nghtv Reference hcein xc any v>t^-'T*t. ^ Dv ^.r^de narno, tf^Oernafk^ mir'ij'iJClureT. or otrierw «d <Jo*^ •nenaalion. or liivONng bv 'Vhe United _^_ . vs and opinions of authors enp'«i«] herein do noi IOM t' irip UnitK* Stales Govefnrneni c a^v agency For presentation at the 11th World Conference of the International Nuclear Target Development Society, October 6-8, 1982, Seattle, Washington. By acceptance of this article, the publisher or recipient acknowledge* the U.S. Govarnment's right to retain a nonaxclusiva, royalty-frtta license in and to any copyright covering tha article. NOTICE P0T09_0£-Jfns,J^SPQRT_JfgE_Il,LESIBLE. I has been reproauce.1 from the best available aopy to pensit the broadest possible avail- ability* *Research sponsored by the Office of Basic Energy Science, U.S. Department of Energy under contract W-7405-eng-26 with the Union Carbide Co rpcration,, DISTRIBUTION OF THIS DOCUMEHT 18 UNLIMITED ' CONCURRENT REDUCTION AND DISTILLATION - AY. IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC H. -

KULAGE-DISSERTATION-2013.Pdf
ENCRYPTED NANOPARTICLES FOR SECURITY, COUNTER-INDUSTRIAL ESPIONAGE, AND COUNTERFEITING A Dissertation by ZACHARY ANDREW KULAGE Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, William S. Charlton Committee Members, Sean M. McDeavitt John W. Poston, Sr. Charles M. Folden III Head of Department, Yassin A. Hassan December 2013 Major Subject: Nuclear Engineering Copyright 2013 Zachary Andrew Kulage ABSTRACT Acts of terrorism and the use of explosives raise concerns about what can be done to prevent such acts. From stopping such a threat to preventing the illicit counterfeiting and smuggling of sensitive and proprietary goods, identification tag- gant technology can be applied to identify and interdict materials of a dubious na- ture. This cost effective approach to tag and track materials will find application in both the government and private sectors, particularly in the prevention of corporate espionage. A taggant system was created based on the modification of the natural isotopic vector of cadmium and tellurium to create artificial, unique, and robust identification capabilities. A proof of concept trial was undertaken to ascertain the effectiveness, reliability, and integrity of a system. Stable isotopes were mixed in various combi- nations to alter the isotopic vector and then neutron activation analysis techniques were used to evaluate the quality of the taggants. Two different neutron activation analysis facilities of differing capabilities were employed to test the limitations of the method in detection of trace amounts of taggant material. Testing of the taggant system found that solid phase taggants were capable of producing around 14,000 unique taggants for under $250 each while liquid phase taggants were capable of producing around 2,000 unique taggants for under $50 each. -

Mass Fractionation Laws, Mass-Independent Effects, and Isotopic Anomalies Nicolas Dauphas and Edwin A
EA44CH26-Dauphas ARI 10 June 2016 9:41 ANNUAL REVIEWS Further Click here to view this article's online features: • Download figures as PPT slides • Navigate linked references • Download citations Mass Fractionation Laws, • Explore related articles • Search keywords Mass-Independent Effects, and Isotopic Anomalies Nicolas Dauphas1,∗ and Edwin A. Schauble2 1Origins Laboratory, Department of the Geophysical Sciences and Enrico Fermi Institute, The University of Chicago, Chicago, Illinois 60637; email: [email protected] 2Department of Earth and Space Sciences, University of California, Los Angeles, California 90095 Annu. Rev. Earth Planet. Sci. 2016. 44:709–83 Keywords First published online as a Review in Advance on isotopes, fractionation, laws, NFS, nuclear, anomalies, nucleosynthesis, May 18, 2016 meteorites, planets The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: Isotopic variations usually follow mass-dependent fractionation, meaning 10.1146/annurev-earth-060115-012157 that the relative variations in isotopic ratios scale with the difference in Copyright c 2016 by Annual Reviews. mass of the isotopes involved (e.g., δ17O ≈ 0.5 × δ18O). In detail, how- All rights reserved ever, the mass dependence of isotopic variations is not always the same, ∗ Corresponding author and different natural processes can define distinct slopes in three-isotope diagrams. These variations are subtle, but improvements in analytical capa- Access provided by University of Chicago Libraries on 07/19/16. For personal use only. Annu. Rev. Earth Planet. Sci. 2016.44:709-783. Downloaded from www.annualreviews.org bilities now allow precise measurement of these effects and make it possi- ble to draw inferences about the natural processes that caused them (e.g., reaction kinetics versus equilibrium isotope exchange).