The Discoverers of the Ruthenium Isotopes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hetc-3Step Calculations of Proton Induced Nuclide Production Cross Sections at Incident Energies Between 20 Mev and 5 Gev
JAERI-Research 96-040 JAERI-Research—96-040 JP9609132 JP9609132 HETC-3STEP CALCULATIONS OF PROTON INDUCED NUCLIDE PRODUCTION CROSS SECTIONS AT INCIDENT ENERGIES BETWEEN 20 MEV AND 5 GEV August 1996 Hiroshi TAKADA, Nobuaki YOSHIZAWA* and Kenji ISHIBASHI* Japan Atomic Energy Research Institute 2 G 1 0 1 (T319-11 l This report is issued irregularly. Inquiries about availability of the reports should be addressed to Research Information Division, Department of Intellectual Resources, Japan Atomic Energy Research Institute, Tokai-mura, Naka-gun, Ibaraki-ken, 319-11, Japan. © Japan Atomic Energy Research Institute, 1996 JAERI-Research 96-040 HETC-3STEP Calculations of Proton Induced Nuclide Production Cross Sections at Incident Energies between 20 MeV and 5 GeV Hiroshi TAKADA, Nobuaki YOSHIZAWA* and Kenji ISHIBASHP* Department of Reactor Engineering Tokai Research Establishment Japan Atomic Energy Research Institute Tokai-mura, Naka-gun, Ibaraki-ken (Received July 1, 1996) For the OECD/NEA code intercomparison, nuclide production cross sections of l60, 27A1, nalFe, 59Co, natZr and 197Au for the proton incidence with energies of 20 MeV to 5 GeV are calculated with the HETC-3STEP code based on the intranuclear cascade evaporation model including the preequilibrium and high energy fission processes. In the code, the level density parameter derived by Ignatyuk, the atomic mass table of Audi and Wapstra and the mass formula derived by Tachibana et al. are newly employed in the evaporation calculation part. The calculated results are compared with the experimental ones. It is confirmed that HETC-3STEP reproduces the production of the nuclides having the mass number close to that of the target nucleus with an accuracy of a factor of two to three at incident proton energies above 100 MeV for natZr and 197Au. -

An Octad for Darmstadtium and Excitement for Copernicium
SYNOPSIS An Octad for Darmstadtium and Excitement for Copernicium The discovery that copernicium can decay into a new isotope of darmstadtium and the observation of a previously unseen excited state of copernicium provide clues to the location of the “island of stability.” By Katherine Wright holy grail of nuclear physics is to understand the stability uncover its position. of the periodic table’s heaviest elements. The problem Ais, these elements only exist in the lab and are hard to The team made their discoveries while studying the decay of make. In an experiment at the GSI Helmholtz Center for Heavy isotopes of flerovium, which they created by hitting a plutonium Ion Research in Germany, researchers have now observed a target with calcium ions. In their experiments, flerovium-288 previously unseen isotope of the heavy element darmstadtium (Z = 114, N = 174) decayed first into copernicium-284 and measured the decay of an excited state of an isotope of (Z = 112, N = 172) and then into darmstadtium-280 (Z = 110, another heavy element, copernicium [1]. The results could N = 170), a previously unseen isotope. They also measured an provide “anchor points” for theories that predict the stability of excited state of copernicium-282, another isotope of these heavy elements, says Anton Såmark-Roth, of Lund copernicium. Copernicium-282 is interesting because it University in Sweden, who helped conduct the experiments. contains an even number of protons and neutrons, and researchers had not previously measured an excited state of a A nuclide’s stability depends on how many protons (Z) and superheavy even-even nucleus, Såmark-Roth says. -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -
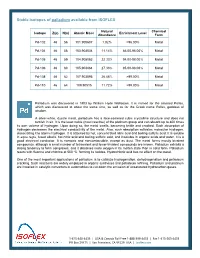
Stable Isotopes of Palladium Available from ISOFLEX
Stable isotopes of palladium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Pd-102 46 56 101.905607 1.02% >96.00% Metal Pd-104 46 58 103.904034 11.14% 86.00-98.00% Metal Pd-105 46 59 104.905083 22.33% 94.00-98.00% Metal Pd-106 46 60 105.903484 27.33% 95.00-98.00% Metal Pd-108 46 62 107.903895 26.46% >99.00% Metal Pd-110 46 64 109.90515 11.72% >99.00% Metal Palladium was discovered in 1803 by William Hyde Wollaston. It is named for the asteroid Pallas, which was discovered at about the same time, as well as for the Greek name Pallas, goddess of wisdom. A silver-white, ductile metal, palladium has a face-centered cubic crystalline structure and does not tarnish in air. It is the least noble (most reactive) of the platinum group and can absorb up to 800 times its own volume of hydrogen. Upon doing so, the metal swells, becoming brittle and cracked. Such absorption of hydrogen decreases the electrical conductivity of the metal. Also, such absorption activates molecular hydrogen, dissociating it to atomic hydrogen. It is attacked by hot, concentrated nitric acid and boiling sulfuric acid. It is soluble in aqua regia, fused alkalis, hot nitric acid and boiling sulfuric acid, and insoluble in organic acids and water. It is a good electrical conductor. It is nontoxic and noncombustible, except as dust. The metal forms mostly bivalent compounds, although a small number of tetravalent and fewer trivalent compounds are known. -

Heavy Element Nucleosynthesis
Heavy Element Nucleosynthesis A summary of the nucleosynthesis of light elements is as follows 4He Hydrogen burning 3He Incomplete PP chain (H burning) 2H, Li, Be, B Non-thermal processes (spallation) 14N, 13C, 15N, 17O CNO processing 12C, 16O Helium burning 18O, 22Ne α captures on 14N (He burning) 20Ne, Na, Mg, Al, 28Si Partly from carbon burning Mg, Al, Si, P, S Partly from oxygen burning Ar, Ca, Ti, Cr, Fe, Ni Partly from silicon burning Isotopes heavier than iron (as well as some intermediate weight iso- topes) are made through neutron captures. Recall that the prob- ability for a non-resonant reaction contained two components: an exponential reflective of the quantum tunneling needed to overcome electrostatic repulsion, and an inverse energy dependence arising from the de Broglie wavelength of the particles. For neutron cap- tures, there is no electrostatic repulsion, and, in complex nuclei, virtually all particle encounters involve resonances. As a result, neutron capture cross-sections are large, and are very nearly inde- pendent of energy. To appreciate how heavy elements can be built up, we must first consider the lifetime of an isotope against neutron capture. If the cross-section for neutron capture is independent of energy, then the lifetime of the species will be ( )1=2 1 1 1 µn τn = ≈ = Nnhσvi NnhσivT Nnhσi 2kT For a typical neutron cross-section of hσi ∼ 10−25 cm2 and a tem- 8 9 perature of 5 × 10 K, τn ∼ 10 =Nn years. Next consider the stability of a neutron rich isotope. If the ratio of of neutrons to protons in an atomic nucleus becomes too large, the nucleus becomes unstable to beta-decay, and a neutron is changed into a proton via − (Z; A+1) −! (Z+1;A+1) + e +ν ¯e (27:1) The timescale for this decay is typically on the order of hours, or ∼ 10−3 years (with a factor of ∼ 103 scatter). -

A Guide to Naturally Occurring Radioactive Materials (NORM)
ABOUT NORM SOME NORM NUCLIDES OF SPECIAL INTEREST A Guide to Naturally There are three types of NORM: Occurring Radioactive • Cosmogenic – NORM produced by cosmic rays interacting with the Earth’s Materials (NORM) Beryllium 7 atmosphere; the most important Developed by the 3 7 14 examples are H (tritium), Be, and C. 7Be is being continuously formed in the DHS Secondary Reachback Program • Primordial – Sufficiently long-lived atmosphere by cosmic rays. Jet engines can March 2010 NORM for some to have survived from accumulate enough 7Be to set off before the formation of the Earth; there radiological alarms when being cleaned. are 20 primordial NORM nuclides with As defined by the International Atomic the most important being 40K, 232Th, Energy Agency (IAEA), Naturally 235U, and 238U. Polonium 210 Occurring Radioactive Materials • Daughters – When 232Th, 235U and 238U In the 238U decay chain 210Po is a distant (NORM) include all natural radioactive decay 42 different radioactive nuclides daughter of 226Ra. 210Po gained notoriety in materials where human activities have are formed (see below) with the most the 1960s as a radioactive trace component increased the potential for exposure in important being 222Rn, 226Ra, 228Ra and in cigarette smoke. Heavy use of phosphate comparison with the unaltered natural 228 Ac. fertilizers (which have trace amounts of out here. fold Next situation. If processing has increased 226Ra) can triple the amount of 210Po found the concentrations of radionuclides in a When 232Th, 235U and 238U decay a in tobacco. material then it is Technologically radioactive “daughter” nuclide is formed, Enhanced NORM (TE-NORM). -

(12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden Et Al
USOO8625731B2 (12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden et al. (45) Date of Patent: Jan. 7, 2014 (54) COMPACT NEUTRONGENERATOR FOR (51) Int. Cl. MEDICAL AND COMMERCIAL SOTOPE G2G 4/02 (2006.01) PRODUCTION, FISSION PRODUCT G2G 4/00 (2006.01) PURIFICATION AND CONTROLLED (52) U.S. Cl. GAMMA REACTIONS FOR DIRECT USPC ............................ 376/108: 376/112:376/156 ELECTRIC POWER GENERATION (58) Field of Classification Search USPC .......................... 376/157, 108,156, 171, 172 (76) Inventors: Charles S. Holden, San Francisco, CA See application file for complete search history. (US); Robert E. Schenter, Portland, OR (US) (56) References Cited *) Notice: Subject to anyy disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 3,748,226 A * 7/1973 Ribe et al. ..................... 376,124 U.S.C. 154(b) by 961 days. 3,778,627 A * 12/1973 Carpenter ...... ... 376, 192 4,749,540 A * 6/1988 Bogartet al. .. ... 376/133 (21) Appl. No.: 12/296,844 4,997,619 A * 3/1991 Pettus ............ ... 376,288 2004/02284.33 A1* 1 1/2004 Magill et al. ... 376/347 (22) PCT Filed: Apr. 13, 2007 2005/022O248 A1* 10, 2005 Ritter ...... ... 376/190 2008/O144762 A1* 6/2008 Holden et al. ..... ... 376/416 (86). PCT No.: PCT/US2OOTAO66668 * cited by examiner S371 (c)(1), (2), (4) Date: Oct. 10, 2008 Primary Examiner — Jack W Keith Assistant Examiner — Sean P Burke (87) PCT Pub. No.: WO2008/060663 (74) Attorney, Agent, or Firm — Craig M. Stainbrook; PCT Pub. Date: May 22, 2008 Stainbrook & Stainbrook, LLP (65) Prior Publication Data (57) ABSTRACT A neutron generator and isotope production apparatus and US 2011 FO268237 A1 Nov. -

Production and Properties Towards the Island of Stability
This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Author(s): Leino, Matti Title: Production and properties towards the island of stability Year: 2016 Version: Please cite the original version: Leino, M. (2016). Production and properties towards the island of stability. In D. Rudolph (Ed.), Nobel Symposium NS 160 - Chemistry and Physics of Heavy and Superheavy Elements (Article 01002). EDP Sciences. EPJ Web of Conferences, 131. https://doi.org/10.1051/epjconf/201613101002 All material supplied via JYX is protected by copyright and other intellectual property rights, and duplication or sale of all or part of any of the repository collections is not permitted, except that material may be duplicated by you for your research use or educational purposes in electronic or print form. You must obtain permission for any other use. Electronic or print copies may not be offered, whether for sale or otherwise to anyone who is not an authorised user. EPJ Web of Conferences 131, 01002 (2016) DOI: 10.1051/epjconf/201613101002 Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements Production and properties towards the island of stability Matti Leino Department of Physics, University of Jyväskylä, PO Box 35, 40014 University of Jyväskylä, Finland Abstract. The structure of the nuclei of the heaviest elements is discussed with emphasis on single-particle properties as determined by decay and in- beam spectroscopy. The basic features of production of these nuclei using fusion evaporation reactions will also be discussed. 1. Introduction In this short review, some examples of nuclear structure physics and experimental methods relevant for the study of the heaviest elements will be presented. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -

Study of the Properties of Hydrogen and Deuterium in Beta Phase Palladium Hydride and Deuteride
Study of the Properties of Hydrogen and Deuterium in Beta Phase Palladium Hydride and Deuteride SIMON ANTHONY STEEL 18/04/2018 Materials and Physics Research Group School of Computing Science & Engineering This thesis is submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy Dedication This work is dedicated to the memory of Professor Donald Keith Ross. “I may not have gone where I intended to go, but I think I have ended up where I needed to be.” Douglas Adams i Contents Dedication .................................................................................................................................... i Figures ...................................................................................................................................... vii Acknowledgements .................................................................................................................... x Glossary .................................................................................................................................... xii Units, Constants, and Standard Values ................................................................................... xiii Conventions in this Document ................................................................................................ xiv Abstract .................................................................................................................................... xv 1 Introduction ........................................................................................................................ -

The Discoverers of the Palladium Isotopes the THIRTY-FOUR KNOWN PALLADIUM ISOTOPES FOUND BETWEEN 1935 and 1997
DOI: 10.1595/147106706X110817 The Discoverers of the Palladium Isotopes THE THIRTY-FOUR KNOWN PALLADIUM ISOTOPES FOUND BETWEEN 1935 AND 1997 By J. W. Arblaster Coleshill Laboratories, Gorsey Lane, Coleshill, West Midlands B46 1JU, U.K.; E-mail: [email protected] This is the fourth in a series of reviews of circumstances surrounding the discoveries of the isotopes of the six platinum group elements. The first review, on platinum isotopes, was published in this Journal in October 2000, the second, on iridium isotopes, was published here in October 2003 and the third, on osmium isotopes, was published in October 2004 (1). The current review looks at the discovery and the discoverers of the thirty-four isotopes of palladium. Of the thirty-four known isotopes of palladium, ment activities found for palladium, such as a half- six occur naturally with the following authorised life of six hours discovered by Fermi et al. in 1934 isotopic abundances (2): (9) and half-lifes of 3 minutes and 60 hours discov- ered by Kurchatov et al. (10) in 1935 do not appear to have been confirmed. The Naturally Occurring Isotopes of Palladium In 1940, Nishima et al. (11) obtained an unspec- Mass number Isotopic abundance, % ified activity with a half-life of 26 minutes which is also likely to have been 111Pd. The actual half-life of 102Pd 1.02 111 104Pd 11.14 Pd is now known to be 23 minutes, so the differ- 105Pd 22.33 ent values obtained above are probably indicative 106 Pd 27.33 of calibration problems. 108 Pd 26.46 These unspecified activities raise problems con- 110Pd 11.72 cerning the precedence for treating each discovery in this paper. -

Properties and Applications of Ruthenium
Chapter 17 Properties and Applications of Ruthenium Anil K. Sahu, Deepak K. Dash, Koushlesh Mishra, Saraswati P. Mishra, Rajni Yadav and Pankaj Kashyap Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.76393 Abstract Ruthenium (Ru) with atomic number of 44 is one of the platinum group metals, the others being Rh, Pd, Os, Ir and Pt. In earth’s crust, it is quite rare, found in parts per billion quantities, in ores containing some of the other platinum group metals. Ruthenium is silvery whitish, lustrous hard metal with a shiny surface. It has seven stable isotopes. Recently, coordination and organometallic chemistry of Ru has shown remarkable growth. In this chapter, we review the application of Ru in diverse fields along with its physical and chemical properties. In the applications part of Ru we have primarily focused on the biomedical applications. The biomedical applications are broadly divided into diagnostic and treatment aspects. Ru and their complexes are mainly used in determination of ferritin, calcitonin and cyclosporine and folate level in human body for diagnosis of diseases. Treat- ment aspects focuses on immunosuppressant, antimicrobial and anticancer activity. Keywords: ruthenium, platinum group, biomedical application, rare element, cancer, isotopes 1. Discovery of ruthenium Ruthenium is one of the 118 chemical elements given in the periodic table. Out of these 118 elements, 92 elements originated from natural sources and remaining 26 elements have been synthesized in laboratories [1, 2]. The last naturally occurring element to be discovered was Uranium in 1789 [1, 3]. Technetium was the first man-made element to be synthesized in the year 1937 [2].