Writing Names and Formulas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Naming Polyatomic Ions and Acids Oxyanions
Oxyanions Naming Polyatomic Ions and Oxyanions Acids Oxyanions- negative ions containing Oxyanions may contain the prefix oxygen. “hypo-”, less than, or “per-”, more than. These have the suffix “-ate” or “-ite” For example - “-ate” means it has more oxygen atoms ClO4 Perchlorate bonded, “-ite” has less - ClO3 Chlorate For example - ClO2 Chlorite 2- SO4 sulfate ClO- Hypochlorite 2- SO3 sulfite Acids Naming acids Naming Acids Certain compounds produce H+ ions in Does it contain oxygen? water, these are called acids. If it does not, it gets the prefix “hydro-” and If it does contain an oxyanion, then the suffix “-ic acid” replace the ending. You can recognize them because the neutral compound starts with “H”. HCl If the ending was “–ate”, add “-ic acid” Hydrochloric acid If the ending was “–ite”, add “-ous acid” For example HCl, H2SO4, and HNO3. HF Don’t confuse a polyatomic ion with a H2SO4 Sulfuric Acid Hydrofluoric acid neutral compound. H2SO3 Sulfurous Acid HCN HCO - is hydrogen carbonate, not an acid. 3 Hydrocyanic acid Examples Examples Nomenclature (naming) of Covalent compounds HNO3 HNO3 Nitric Acid HI HI Hydroiodic acid H3AsO4 H3AsO4 Arsenic Acid HClO2 HClO2 Chlorous Acid 1 Determining the type of bond Covalent bonding is very Covalent bonding is very First, determine if you have an ionic different from ionic naming compound or a covalent compound. similar to ionic naming A metal and a nonmetal will form an You always name the one that is least Ionic names ignored the subscript ionic bond. electronegative first (furthest from because there was only one possible Compounds with Polyatomic ions form fluorine) ratio of elements. -
Carbon Dioxide - Made by FILTERSORB SP3
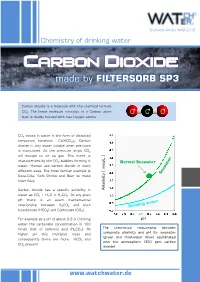
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Acetate C2H3O2 Or CH3COO Or CH3CO2 Ammonium NH4
Name:_____________________ Memorization For AP Chem Polyatmic Ions AP Chemistry Polyatomic Ion list - - - Acetate C2H3O2 or CH 3COO or CH 3CO 2 + Ammonium NH 4 - Ammonium Carbonate NH 4CO 3 Cyanide CN - 2- Carbonate CO 3 2- Dichromate Cr 2O7 - Dihydrogen phosphate H2PO 4 - Hydrogen carbonate HCO 3 - Hydrogen sulfate HSO 4 - Hydronium H3O Hydroxide OH - 2- Monohydrogen Phosphate HPO 4 - Nitrate NO 3 - Nitrite NO 2 2- Oxalate C2O4 - Permanganate MnO 4 3- Phosphate PO 4 2- Sulfate SO 4 2- Sulfite SO 3 Hypochlorite ClO - - Chlorite ClO 2 - Chlorate ClO 3 - Perchlorate ClO 4 2- Chromate CrO 4 2- Silicate SiO 3 Thiocyanate SCN - 2- Thiosulfate S2O3 - Bromate BrO 3 2- Selenate SeO 4 - Bisulfite HSO 3 Name:_____________________ Memorization For AP Chem Rules for Naming an Acid When the name of the anion ends in –ide, the acid name begins with the prefix hydro-, the stem of the anion has the suffix –ic and it is followed by the word acid. -ide becomes hydro _____ic Acid Cl- is the Chloride ion so HCl = hydrochloric acid When the anion name ends in –ite, the acid name is the stem of the anion with the suffix –ous, followed by the word acid. -ite becomes ______ous Acid - ClO 2 is the Chlorite ion so HClO 2 = Chlorous acid. When the anion name ends in –ate, the acid name is the stem of the anion with the suffix –ic, followed by the word acid. -ate becomes ______ic Acid - ClO 3 is the Chlorate ion so HClO 3 = Chloric acid. Rules for Naming Ionic Compounds 1. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

H2CS) and Its Thiohydroxycarbene Isomer (HCSH
A chemical dynamics study on the gas phase formation of thioformaldehyde (H2CS) and its thiohydroxycarbene isomer (HCSH) Srinivas Doddipatlaa, Chao Hea, Ralf I. Kaisera,1, Yuheng Luoa, Rui Suna,1, Galiya R. Galimovab, Alexander M. Mebelb,1, and Tom J. Millarc,1 aDepartment of Chemistry, University of Hawai’iatManoa, Honolulu, HI 96822; bDepartment of Chemistry and Biochemistry, Florida International University, Miami, FL 33199; and cSchool of Mathematics and Physics, Queen’s University Belfast, Belfast BT7 1NN, Northern Ireland, United Kingdom Edited by Stephen J. Benkovic, The Pennsylvania State University, University Park, PA, and approved August 4, 2020 (received for review March 13, 2020) Complex organosulfur molecules are ubiquitous in interstellar molecular sulfur dioxide (SO2) (21) and sulfur (S8) (22). The second phase clouds, but their fundamental formation mechanisms have remained commences with the formation of the central protostars. Tempera- largely elusive. These processes are of critical importance in initiating a tures increase up to 300 K, and sublimation of the (sulfur-bearing) series of elementary chemical reactions, leading eventually to organo- molecules from the grains takes over (20). The subsequent gas-phase sulfur molecules—among them potential precursors to iron-sulfide chemistry exploits complex reaction networks of ion–molecule and grains and to astrobiologically important molecules, such as the amino neutral–neutral reactions (17) with models postulating that the very acid cysteine. Here, we reveal through laboratory experiments, first sulfur–carbon bonds are formed via reactions involving methyl electronic-structure theory, quasi-classical trajectory studies, and astro- radicals (CH3)andcarbene(CH2) with atomic sulfur (S) leading to chemical modeling that the organosulfur chemistry can be initiated in carbonyl monosulfide and thioformaldehyde, respectively (18). -

Ambient Water Quality Criteria for Polycyclic Aromatic Hydrocarbons (Pahs)
Ambient Water Quality Criteria For Polycyclic Aromatic Hydrocarbons (PAHs) Ministry of Environment, Lands and Parks Province of British Columbia N. K. Nagpal, Ph.D. Water Quality Branch Water Management Division February, 1993 ACKNOWLEDGEMENTS The author is indebted to the following individual and agencies for providing valuable comments during the preparation of this document. Dr. Ray Copes BC. Ministry of Health, Victoria, BC. Dr. G. R. Fox Environmental Protection Div., BC. MOELP, Victoria, BC. Mr. L. W. Pommen Water Quality Branch, BC. MOELP, Victoria, BC. Mr. R. J. Rocchini Water Quality Branch, BC. MOELP, Victoria, BC. Ms. Sherry Smith Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Mr. Scott Teed Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Ms. Bev Raymond Integrated Programs Branch, Inland Waters, Environment Canada, North Vancouver, BC. 1.0 INTRODUCTION Polycyclic aromatic hydrocarbons (PAHs) are organic compounds which are non- essential for the growth of plants, animals or humans; yet, they are ubiquitous in the environment. When present in sufficient quantity in the environment, certain PAHs are toxic and carcinogenic to plants, animals and humans. This document discusses the characteristics of PAHs and their effects on various water uses, which include drinking Ministry of Environment Water Protection and Sustainability Branch Mailing Address: Telephone: 250 387-9481 Environmental Sustainability PO Box 9362 Facsimile: 250 356-1202 and Strategic Policy Division Stn Prov Govt Website: www.gov.bc.ca/water Victoria BC V8W 9M2 water, aquatic life, wildlife, livestock watering, irrigation, recreation and aesthetics, and industrial water supplies. A significant portion of this document discusses the effects of PAHs upon aquatic life, due to its sensitivity to PAHs. -

Monoatomic Ions You Should Know
Monoatomic ions you should know Monoatomic Cations Elements that form a single cation Elements that form two or more cations Ion Name Ion Name Common Name + 2+ H hydrogen ion /proton Cr chromium(II) ion Li+ lithium ion Cr3+ chromium(III) ion + 2+ Na sodium ion Fe iron(II) ion ferrous ion + 3+ K potassium ion Fe iron(III) ion ferric ion + 2+ Rb rubidium ion Ni nickel(II) + 3+ Cs cesium ion Ni nickel(III) 2+ + Be beryllium ion Cu copper(I) ion cuprous ion 2+ 2+ Mg magnesium ion Cu copper(II) ion cupric ion 2+ 2+ Ca calcium ion Hg2 mercury(I) ion* mercurous ion Sr2+ strontium ion Hg2+ mercury(II) ion mercuric ion Ba2+ barium ion Sn2+ tin(II) ion stannous ion Al3+ aluminum ion Sn4+ tin(IV) ion stannic ion Zn2+ zinc ion Pb2+ lead(II) ion plumbous ion Ag+ silver ion Pb4+ lead(IV) ion plumbic ion Cd2+ cadmium ion *this is actually a polyatomic ion Monoatomic Anions (all form only single ion) Ion Name Ion Name N3- nitride ion H- hydride ion P3- phosphide ion F- fluoride ion O2- oxide ion Cl- chloride ion S2- sulfide ion Br- bromide ion I- iodide ion Polyatomic ions you should know Formula Name Formula Name - 2- C2H3O2 acetate ion CO3 carbonate ion + - NH4 ammonium ion HCO3 bicarbonate ion 2- 2- CrO4 chromate ion SO4 sulfate ion Cr O 2- dichromate ion HSO - bisulfate ion 2 7 4 - 2- CN cyanide ion SO3 sulfite ion - - HO hydroxide ion HSO3 bisulfite ion 2- - C2O4 oxalate ion ClO4 perchlorate ion - - MnO4 permanganate ion ClO3 chlorate ion - - NO3 nitrate ion ClO2 chlorite ion - - NO2 nitrite ion ClO hypochlorite ion PO 3- phosphate ion 4 2- HPO4 hydrogen phosphate ion - H2PO4 dihydrogen phosphate ion Acids you should know Hydrohalic acids Oxygen containing acids Formula Name Formula Name HF hydrofluoric acid H3PO4 phosphoric acid HCl hydrochloric acid H3PO3 phosphorous acid HBr hydrobromic acid H2SO4 sulfuric acid HI hydroiodic acid H2SO3 sulfurous acid H2CO3 carbonic acid HMnO4 permanganic acid HNO3 nitric acid HNO2 nitrous acid HC2H3O2 acetic acid H2C2O4 oxalic acid HClO4 perchloric acid HClO3 chloric acid HClO2 chlorous acid HClO hypochlorous acid . -

The Life and Times of Carbon, Student Workbook
ELL Chemistry Assignment # 2 Name: __________________________________ Period: Teacher:_____________________ Collecting Carbon Lesson 1 | page 1 of 4 Where Can We Find Carbon? Carbon compounds are take up carbon dioxide from three-fourths of the dry weight everywhere around us. They the air, and use photosynthesis of plants is carbohydrates. In are in our foods, plastics, soils, to turn carbon dioxide into other words, when you look at in water, in the air, and in our carbohydrates. Carbohydrates plant matter (the stem, leaves, bodies. Carbon compounds can are carbon compounds that roots, and grains) you are come in three physical forms: store energy in the forms we call looking largely at carbohydrates. gas, liquid, and solid. Plants “starches” and “sugars.” About Animals (including humans) get their carbohydrates by eating plants, but they do not store much of what they consume. In fact, less than 1% of the body weight of animals is made up of carbohydrates. The main forms of carbon are: nonliving (abiotic) in rocks, soils, and sediments, and in water, such as bicarbonate, carbonate; living (biotic), such as plant and animal matter and dead organic matter; and carbon-based gases, such as carbon dioxide (CO2), methane (CH4), and carbon monoxide (CO). Chemists use a special system to describe compounds. They use a chemical formula of symbols that indicate the elements that make up chemical compounds. A chemical formula is also called a “molecular formula.” The chemical formula of carbon dioxide is CO2 indicating one carbon atom bonded to two oxygen atoms. The symbol for carbon is C; for hydrogen is H; for oxygen is O; for silica is Si. -

Significant Figures
For students and parents/guardians Table of Contents In the Elements Handbook, you’ll find use- ful information about the properties of the Elements Handbook . 901 main group elements from the periodic table. Hydrogen. 904 You’ll also learn about real-world applications Group 1: Alkali Metals. 906 for many of the elements. Group 2: Alkaline Earth Metals . 910 The Math Handbook helps you review and Groups 3–12: Transition Elements . 916 sharpen your math skills so you get the most Group 13: Boron Group . 922 out of understanding how to solve math prob- Group 14: Carbon Group . .926 lems involving chemistry. Reviewing the rules Group 15: Nitrogen Group . 932 Group 16: Oxygen Group . .936 for mathematical operations such as scientific Group 17: Halogen Group . 940 notation, fractions, and logarithms can also Group 18: Noble Gases . .944 help you boost your test scores. The reference tables are another tool that Math Handbook . .946 will assist you. The practice problems and Scientific Notation . .946 solutions are resources that will help increase Operations with Scientific Notation . 948 your comprehension. Square and Cube Roots . 949 Significant Figures . .949 Solving Algebraic Equations. 954 Dimensional Analysis . 956 Unit Conversion . 957 Drawing Line Graphs. 959 Using Line Graphs . .961 Ratios, Fractions, and Percents. 964 Operations Involving Fractions . .965 Logarithms and Antilogarithms. 966 Reference Tables. 968 R-1 Color Key. 968 R-2 Symbols and Abbreviations. 968 R-3 Solubility Product Constants . 969 R-4 Physical Constants . .969 R-5 Names and Charges of Polyatomic Ions . 970 R-6 Ionization Constants . .970 R-7 Properties of Elements.