ASN Kidney Week 2017 Disclosures 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2017 Match Day Results by Program
Class of 2017 Match Results Anesthesiology New York Presbyterian Hospital-Columbia University Medical Center University of Illinois College of Medicine-Chicago University of Texas Medical School-Houston Icahn School of Medicine/St Luke's-Roosevelt Hospital Center (New York) University of Florida College of Medicine-Shands Hospital New York Presbyterian Hospital-Weill Cornell Medical Center Einstein/Montefiore Medical Center (New York) New York Presbyterian Hospital-Columbia University Medical Center Dermatology University at Buffalo School of Medicine (New York) University of Buffalo School of Medicine (New York) Cleveland Clinic Foundation (OH) Emergency Medicine Einstein/Montefiore Medical Center (New York) University of Massachusetts Medical School Staten Island University Hospital (New York) Stanford University Programs (California) Stony Brook Teaching Hospitals (New York) New York Hospital Medical Center Queens (New York) Eastern Virginia Medical School University of Washington Affiliated Hospitals Icahn School of Medicine/St Luke's-Roosevelt Hospital Center (New York) University of Connecticut School of Medicine Rhode Island Hospital/Brown University Wake Forest Baptist Medical Center (North Carolina) Icahn School of Medicine/St Luke's-Roosevelt Hospital Center (New York) Einstein/Montefiore Medical Center (New York) Oregon Health and Science University Dartmouth-Hitchcock Medical Center (New Hampshire) Einstein/Montefiore Medical Center (New York) University of Washington Affiliated Hospitals Einstein/Montefiore Medical Center -
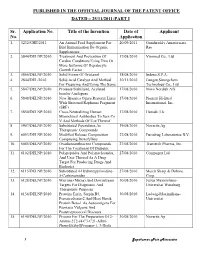
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

Residency Placement Location by Specialty for the Class of 2017
RESIDENCY PLACEMENT LOCATION BY SPECIALTY FOR THE CLASS OF 2017 ANESTHESIOLOGY Program Location Type Detroit Medical Center/WSU Detroit, MI ACGME Drexel U COM/Hahnemann U Hospital Philadelphia, PA ACGME Duke University Medical Center Durham, NC ACGME Geisinger Health System Danville, PA ACGME Jackson Memorial Hospital Miami, FL ACGME Johns Hopkins University MC Baltimore, MD ACGME Maimonides Medical Center Brooklyn, NY ACGME Massachusetts General Hospital Boston, MA ACGME Medical University of South Carolina Charleston, SC ACGME Montefiore Medical Center/Einstein Bronx, NY ACGME NYMC-Westchester Medical Center Valhalla, NY ACGME Oklahoma State University MC Tulsa, OK AOA Rush University Medical Center (2) Chicago, IL ACGME Rutgers-RW Johnson Medical School New Brunswick, NJ ACGME SUNY HSC Brooklyn (3) Brooklyn, NY ACGME SUNY Upstate Medical University Syracuse, NY ACGME U of Connecticut School of Medicine (2) Farmington, CT ACGME U of Rochester/Strong Memorial (3) Rochester, NY ACGME U of Texas Health Science Center San Antonio, TX ACGME U of Texas Medical School Houston, TX ACGME U of Washington Affiliate Hospitals Seattle, WA ACGME CHILD NEUROLOGY Program Location Type SUNY HSC Brooklyn Brooklyn, NY ACGME DERMATOLOGY Program Location Type St. John’s Episcopal Far Rockaway, NY AOA DIAGNOSTIC RADIOLOGY Program Location Type Bryn Mawr Hospital Bryn Mawr, PA ACGME Morristown Memorial Hospital Morristown, NJ ACGME Nassau University Medical Center East Meadow, NY ACGME Stony Brook Teaching Hospitals Stony Brook, NY ACGME UPMC Medical Education -

The History of the First Kidney Transplantation
165+3 14 mm "Service to society is the rent we pay for living on this planet" The History of the Joseph E. Murray, 1990 Nobel-laureate who performed the first long-term functioning kidney transplantation in the world First Kidney "The pioneers sacrificed their scientific life to convince the medical society that this will become sooner or later a successful procedure… – …it is a feeling – now I am Transplantation going to overdo - like taking part in creation...” András Németh, who performed the first – a European Overview Hungarian renal transplantation in 1962 E d i t e d b y : "Professor Langer contributes an outstanding “service” to the field by a detailed Robert Langer recording of the history of kidney transplantation as developed throughout Europe. The authoritative information is assembled country by country by a generation of transplant professionals who knew the work of their pioneer predecessors. The accounting as compiled by Professor Langer becomes an essential and exceptional reference document that conveys the “service to society” that kidney transplantation has provided for all mankind and that Dr. Murray urged be done.” Francis L. Delmonico, M.D. Professor of Surgery, Harvard Medical School, Massachusetts General Hospital Past President The Transplantation Society and the Organ Procurement Transplant Network (UNOS) Chair, WHO Task Force Organ and Tissue Donation and Transplantation The History of the First Kidney Transplantation – a European Overview European a – Transplantation Kidney First the of History The ISBN 978-963-331-476-0 Robert Langer 9 789633 314760 The History of the First Kidney Transplantation – a European Overview Edited by: Robert Langer SemmelweisPublishers www.semmelweiskiado.hu Budapest, 2019 © Semmelweis Press and Multimedia Studio Budapest, 2019 eISBN 978-963-331-473-9 All rights reserved. -

Newsletteralumni News of the Newyork-Presbyterian Hospital/Columbia University Department of Surgery Volume 13, Number 1 Summer 2010
NEWSLETTERAlumni News of the NewYork-Presbyterian Hospital/Columbia University Department of Surgery Volume 13, Number 1 Summer 2010 CUMC 2007-2009 Transplant Activity Profile* Activity Kidney Liver Heart Lung Pancreas Baseline list at year start 694 274 174 136 24 Deceased donor transplant 123 124 93 57 11 Living donor transplant 138 17 — 0 — Transplant rate from list 33% 50% 51% 57% 35% Mortality rate while on list 9% 9% 9% 15% 0% New listings 411 217 144 68 23 Wait list at year finish 735 305 204 53 36 2007-June 2008 Percent 1-Year Survival No % No % No % No % No % Adult grafts 610 91 279 86 169 84 123 89 6 100 Adult patients 517 96 262 88 159 84 116 91 5 100 Pediatric grafts 13 100 38 86 51 91 3 100 0 — Pediatric patients 11 100 34 97 47 90 2 100 0 — Summary Data Total 2009 living donor transplants 155 (89% Kidney) Total 2009 deceased donor transplants 408 (30% Kidney, 30% Liver) 2007-June 2008 adult 1-year patient survival range 84% Heart to 100% Pancreas 2007-June 2008 pediatric 1-year patient survival range 90% Heart to 100% Kidney or lung *Health Resource and Service Administration’s Scientific Registry of Transplant Recipients (SRTR) Ed Note. The figure shows the US waiting list for whole organs which will only be partially fulfilled by some 8,000 deceased donors, along with 6,600 living donors, who will provide 28,000 to 29,000 organs in 2010. The Medical Center’s role in this process is summarized in the table, and the articles that follow my note expand on this incredible short fall and its potential solutions. -

2019 Greater Bridgeport Region Bridgeport Hospital and St. Vincent's Medical Center Collaborative Community Health Needs Asse
2019 Greater Bridgeport Region Bridgeport Hospital and St. Vincent’s Medical Center Collaborative Community Health Needs Assessment and Implementation Plan By the Health Improvement Alliance This document is a special section of the Fairfield County Community Wellbeing Index 2019, a core program of DataHaven (ctdatahaven.org), in partnership with Fairfield County’s Community Foundation and a Community Health Needs Assessment for the towns served by all Fairfield County hospitals including Bridgeport Hospital and St. Vincent’s Medical Center 1 | Page ABOUT THIS REPORT This document is a special section of the Fairfield County Community Wellbeing Index 2019 (Appendix A), a comprehensive report about Fairfield County and the towns within it. The Community Index was produced by DataHaven in partnership with Fairfield County’s Community Foundation and many other regional partners, including the Health Improvement Alliance (HIA), a coalition serving towns in the Greater Bridgeport region. This document serves as the Community Health Needs Assessment for the six towns in the HIA area (Bridgeport, Easton, Fairfield, Monroe, Stratford, and Trumbull). The Community Health Needs Assessment documents the process that the HIA used to conduct the regional health assessment and health improvement activities. You may find the full Community Wellbeing Index attached to this section, or posted on the DataHaven, Fairfield County’s Community Foundation, Bridgeport Hospital, St. Vincent’s Medical Center, or any of the town health department websites. The Community Health Needs Assessment and Community Health Improvement Plan were approved by the Board of Trustees for St. Vincent’s Medical Center in June 13, 2019 and the Board of Trustees for Bridgeport Hospital in July 9, 2019. -

A Year of Impact Efficiency
A Year of A Year UNITED HOSPITAL FUND UNITED HOSPITAL coverage and access ANNUAL REPORT 2019 REPORT ANNUAL Impact quality and efficiency clinical-community partnerships OFFICERS AND DIRECTORS IMPROVING HEALTH CARE FOR EVERY NEW YORKER Officers John C. Simons Chairman United Hospital Fund works to build a more effective health Anthony Shih, MD, MPH care system for every New Yorker. An independent, nonprofit President organization, we analyze public policy to inform decision- Jo Ivey Boufford, MD Frederick W. Telling, PhD makers, find common ground among diverse stakeholders, Vice Chairmen and develop and support innovative programs that improve the Sheila M. Abrams quality, accessibility, affordability, and experience of patient care. Treasurer Sheila M. Abrams Sally J. Rogers chairman Chad Shearer Senior Vice Presidents Amanda A. Williams Corporate Secretary TABLE OF CONTENTS Directors 1 From the Chairman Stephen Berger Lori Evans Bernstein 2 From the President Jo Ivey Boufford, MD Dale C. Christensen, Jr. 4 Coverage and Access J. Barclay Collins II Robert S. Galvin, MD 6 Quality and Efficiency Jennifer L. Howse, PhD from the Eugene J. Keilin 8 Clinical-Community Partnerships Cary A. Kravet Josh N. Kuriloff 10 UHF Grantmaking Meera Mani, MD, PhD Howard P. Milstein 11 UHF Publications 2019 Susana R. Morales, MD Robert C. Osborne 12 Financial Summary Seun Salami Anthony Shih, MD, MPH 13 Contributors John C. Simons Eileen M. Sullivan-Marx, 16 Staff PhD, RN Frederick W. Telling, PhD Mary Beth C. Tully Barbara A. Yastine Honorary Directors Rev. Dr. John E. Carrington John K. Castle Timothy C. Forbes Barbara P. Gimbel Michael R. Golding, MD Michael A. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

Cooperating Saves Lives Start Contents
Annual Report 2019 Cooperating saves lives start contents Contents Foreword 1. The Eurotransplant community 2. Eurotransplant: donation, allocation, transplantation and waiting lists This document is optimized for Acrobat Reader for best viewing 3. Report of the Board and the central office experience. 4. Histocompatibility Testing Download Acrobat Reader 5. Reporting of non-resident transplants in Eurotransplant 6. Transplant programs and their delegates in 2019 A high resolution version of this document is also available. 7. Scientific output in 2019 Download high resolution pdf 8. Eurotransplant personnel related statistics 9. Abbreviated financial statements All rights reserved. No part of this publication may be reproduced, stored in a retrieval system List of abbreviations or transmitted, in any form or by any means, electronic, mechanical, photocopying or elsewise, without prior permission of Eurotransplant. For permissions, please contact: [email protected] start contents Foreword Dear reader, We are proud to offer you the 2019, digital edition of the International organ exchange Eurotransplant Annual Report. In this environmentally In 2019, 6981 organs from 2042 deceased donors were friendly, digital report you can easily browse via the used for transplantation for patients on the waiting top menu. Weblinks are added to facilitate in finding list of Eurotransplant. This decrease of the number of more specific information on relevant websites. The reported donors is 5,5% compared to 2018 (2159). report provides an overview of the key statistics on 21.5% of organs were exchanged cross-border between organ donation, allocation and transplantation in all the Eurotransplant member states. Thanks to this Eurotransplant countries. international exchange, a suitable donor organ could be You can also read in the report activities within found for many patients in the different Eurotransplant Eurotransplant that took place, decisions that were member states. -

The Rogosin Institute 505 East 70Th Street New York, NY 10021 an Open-Label, Phase II Efficacy Trial of the Implantation Of
The Rogosin Institute Protocol No. 0911010739 ®® ® The Rogosin Institute 505 East 70th Street New York, NY 10021 An Open-Label, Phase II Efficacy Trial of the Implantation of Mouse Renal Adenocarcinoma Cell-Containing Agarose-Agarose Macrobeads in the Treatment of Patients with Treatment-Resistant, Metastatic Pancreatic Adenocarcinoma or Colorectal Cancer Clinical Study Protocol Number: 0911010739 IND Number: BB-IND 10091 November 12th 2014 Amendment 11 CONFIDENTIAL This document and its contents are the property of and confidential to The Rogosin Institute. Any unauthorized copying or use of this document is prohibited. Version: Amendment 11 CONFIDENTIAL Page 1 of 77 November 12th 2014 The Rogosin Institute Protocol No. 0911010739 PROTOCOL SYNOPSIS TITLE OF An Open-Label, Phase II Efficacy Trial of the Implantation of Mouse Renal STUDY Adenocarcinoma Cell-Containing Agarose-Agarose Macrobeads in the Treatment of Patients with Treatment-Resistant, Metastatic Pancreatic Adenocarcinoma or Colorectal Cancer Investigators/ Thomas J. Fahey,III, MD Study Centers: Nataniel Berman, MD Weill Cornell Medicine/The Rogosin Institute 505/520 East 70th Street. New York, NY 10021 Objectives: The primary efficacy outcome for colorectal cancer is post-implantation all- cause mortality, where time to death is defined as the time from the first scan showing disease progression after completion of prior treatment (time of origin, T0) to death from any cause. The primary objective for pancreatic cancer is to determine the Response Rate (RR), at 2 weeks, 4 weeks, 8 weeks, 3 months, 6 months, 9 months, 12 months or longer as possible, of subjects treated with macrobeads after they have failed standard chemotherapeutic regimens or have decided not to pursue standard or experimental chemotherapy for treatment-resistant, metastatic pancreatic adenocarcinoma. -

Analysis of the Trend Over Time of High-Urgency Liver Transplantation Requests in Italy in the 4-Year Period 2014-2017
Analysis of the Trend Over Time of High-Urgency Liver Transplantation Requests in Italy in the 4-Year Period 2014-2017 S. Trapani*, F. Puoti, V. Morabito, D. Peritore, P. Fiaschetti, A. Oliveti, M. Caprio, L. Masiero, L. Rizzato, L. Lombardini, A. Nanni Costa, and M. Cardillo Italian National Transplant Center, Italian Institute of Health, Rome, Italy ABSTRACT Background. The national protocol for the handling of high-urgency (HU) liver organ procurement for transplant is administered by the Italian National Transplant Center. In recent years, we have witnessed a change in requests to access the program. We have therefore evaluated their temporal trend, the need to change the access criteria, the percentage of transplants performed, the time of request satisfaction, and the follow-up. Methods. We analyzed all the liver requests for the HU program received during the 4-year period of 2014 to 2017 for adult recipients (18 years of age): all the variables linked to the recipient or to the donor and the organ transplants are registered in the Informative Transplant System as established by the law 91/99. In addition, intention to treat (ITT) survival rates were compared among 4 different groups: (1) patients on standard waiting lists vs (2) patients on urgency waiting lists, and (3) patients with a history of transplant in urgency vs (4) patients with a history of transplant not in urgency. Results. Out of the 370 requests included in the study, 291 (78.7%) were satisfied with liver transplantation. Seventy-nine requests (21.3%) have not been processed, but if we consider only the real failures, this percentage falls to 13.1% and the percentage of satisfied requests rises to 86.9%. -

1 Early Use of Telehealth in Home Dialysis During the COVID-19
Kidney360 Publish Ahead of Print, published on April 28, 2020 as doi:10.34067/KID.0001662020 Early Use of Telehealth in Home Dialysis During the COVID-19 Pandemic in New York City Vesh Srivatana1,2, Frank Liu1,2, Daniel M. Levine2, Sean D. Kalloo3 1. Division of Nephrology and Hypertension, Weill Cornell Medicine, 525 E. 68th St. New York, NY 10065 2. The Rogosin Institute, 505 East 70th St. Suite 140, New York, NY 10021 3. Division of Nephrology, Columbia University Irving Medical Center, 622 W 168th St, New York, NY 10032 Correspondence: Vesh Srivatana New York Presbyterian Hospital Weill Cornell Medicine Nephrology and Hypertension 505 E 70th St Suite 140 New York City, New York 10021 United States [email protected] 1 Copyright 2020 by American Society of Nephrology. Introduction: The current Coronavirus disease 2019 (COVID-19) pandemic is forcing unprecedented changes in daily life in the United States. During this time many patients are under “lockdown” or “shelter-in-place” orders and the nephrology community has been forced to adapt quickly to this new reality. New York City is the epicenter of the American outbreak and dialysis patients are at especially high risk of COVID-19 exposure due to widespread use of public transportation and densely packed clinical spaces. Here we report the early use and success of using telehealth visits in place of usual in person monthly comprehensive visits in an effort to provide excellent patient care while limiting the risk of patient and staff exposure to COVID-19. Telehealth has distinct advantages during this crisis for the vulnerable home dialysis population, however many practical challenges remain.