Product Information Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Single-Molecule Spectroscopy and Imaging of Biomolecules in Living Cells
Anal. Chem. 2010, 82, 2192–2203 Single-Molecule Spectroscopy and Imaging of Biomolecules in Living Cells Samuel J. Lord, Hsiao-lu D. Lee, and W. E. Moerner* Department of Chemistry, Stanford University, Stanford, California 94305-5080 The number of reports per year on single-molecule imaging instance, the shape of the distribution may be skewed or reveal experiments has grown roughly exponentially since the first multiple subpopulations, which may offer insight into underlying successful efforts to optically detect a single molecule were mechanisms. Each single molecule is a local reporter on the completed over two decades ago. Single-molecule spectros- makeup and conditions of its immediate surroundings, its “na- copy has developed into a field that includes a wealth of noenvironment”, and thus acts as a readout of spatial heterogene- experiments at room temperature and inside living cells. The ity of a sample. SMS also measures time-dependent processes that fast growth of single-molecule biophysics has resulted from are not necessarily synchronized throughout the sample or its benefits in probing heterogeneous populations, one population. For example, multiple catalytic states of an enzyme molecule at a time, as well as from advances in microscopes will be convolved with all the states of other copies in an ensemble, and detectors. This Perspective summarizes the field of live- whereas a SMS experiment can measure uncorrelated stochastic cell imaging of single biomolecules. transitions of a single enzyme. SMS also has the ability to observe intermediate states or rare events, given that the instruments have Single-molecule biophysics spans a range of experiments, from sufficient time resolution. -

Insights on the Interaction of Calcein with Calcium Carbonate and Its Implications in Biomineralization Studies
Insights on the interaction of calcein with calcium carbonate and its implications in biomineralization studies Giulia Magnabosco a,*, Iryna Polishchuk b, Jonathan Erez c, Simona Fermani a, Boaz Pokroy b, Giuseppe Falini a,* The effects of calcein, a marker commonly used to assess entrapment of calcein within the crystal structure of calcium mineral growth in calcifying organism, on calcite and aragonite carbonates is known to occur due its chemical structure.21,22 structure have been investigated. Calcein is entrapped within In this work, we examined in vitro the effect of calcein on the calcite and aragonite and modifies the shape and morphology growth of calcite and aragonite crystals, the two main 2+ of both polymorphs. Moreover, in the presence of Mg , it polymorphs of CaCO3 found in living organisms, using the inhibits aragonite formation in favor of magnesium calcite. calcein concentration in the range usually adopted for in vivo labelling. Studying and understanding of the biomineralization processes For this goal, we used the vapor diffusion method, consisting employ various methods to mark the growth of the inorganic in the diffusion of NH3(g) and CO2(g) obtained from the 2+ components of the organisms, allowing researchers to decomposition of (NH4)2CO3(s), into a Ca solution containing correlate a particular region of the skeleton to the instant of its the dye. This method is relevant for biomineralization process 2- deposition. A common method is to use fluorescent molecules due to the slow increase of the concentration of CO3 ions in to label a particular stage of the deposition process.1 Calcein, a the crystallization solution. -

Rational Design of Small Molecule Fluorescent Probes for Biological Applications
Organic & Biomolecular Chemistry Rational Design of Small Molecule Fluorescent Probes for Biological Applications Journal: Organic & Biomolecular Chemistry Manuscript ID OB-REV-06-2020-001131.R1 Article Type: Review Article Date Submitted by the 13-Jul-2020 Author: Complete List of Authors: Jun, Joomyung; University of Pennsylvania, Chemistry; Massachusetts Institute of Technology, Chemistry Chenoweth, David; University of Pennsylvania, Department of Chemistry Petersson, E.; University of Pennsylvania, Chemistry Page 1 of 16 Organic & Biomolecular Chemistry ARTICLE Rational Design of Small Molecule Fluorescent Probes for Biological Applications a,b a a,c Received 00th January 20xx, Joomyung V. Jun, David M. Chenoweth* and E. James Petersson* Accepted 00th January 20xx Fluorescent small molecules are powerful tools for visualizing biological events, embodying an essential facet of chemical DOI: 10.1039/x0xx00000x biology. Since the discovery of the first organic fluorophore, quinine, in 1845, both synthetic and theoretical efforts have endeavored to “modulate” fluorescent compounds. An advantage of synthetic dyes is the ability to employ modern organic chemistry strategies to tailor chemical structures and thereby rationally tune photophysical properties and functionality of the fluorophore. This review explores general factors affecting fluorophore excitation and emission spectra, molar absorption, Stokes shift, and quantum efficiency; and provides guidelines for chemist to create novel probes. Structure- property relationships concerning the substituents are discussed in detail with examples for several dye families. Then, we present a survey of functional probes based on PeT, FRET, and environmental or photo-sensitivity, focusing on representative recent work in each category. We believe that a full understanding of dyes with diverse chemical moieties enables the rational design of probes for the precise interrogation of biochemical and biological phenomena. -

Recent Advances in Chemical Biology Using Benzophenones and Diazirines As Radical Precursors
molecules Review Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors 1,2, , 1,2, Muhammad Murtaza Hassan * y and Olasunkanmi O. Olaoye y 1 Department of Chemical and Physical Sciences, University of Toronto Mississauga, 3359 Mississauga Road North, Mississauga, ON L5L 1C6, Canada; [email protected] 2 Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON M5S 3H6, Canada * Correspondence: [email protected]; Tel.: +1-905-569-4588 These authors contributed equally to this work. y Academic Editor: Edward Lee-Ruff Received: 18 April 2020; Accepted: 9 May 2020; Published: 13 May 2020 Abstract: The use of light-activated chemical probes to study biological interactions was first discovered in the 1960s, and has since found many applications in studying diseases and gaining deeper insight into various cellular mechanisms involving protein–protein, protein–nucleic acid, protein–ligand (drug, probe), and protein–co-factor interactions, among others. This technique, often referred to as photoaffinity labelling, uses radical precursors that react almost instantaneously to yield spatial and temporal information about the nature of the interaction and the interacting partner(s). This review focuses on the recent advances in chemical biology in the use of benzophenones and diazirines, two of the most commonly known light-activatable radical precursors, with a focus on the last three years, and is intended to provide a solid understanding of their chemical and biological principles and their applications. Keywords: photoaffinity labelling; benzophenone; diazirine; radical precursors; interactome; SABRE; hyperpolarizing agents; crosslinking; photochemistry 1. Introduction The use of radicals or photoactivatable radical precursors has become ubiquitous in the fields of medicinal chemistry and chemical biology in the past three decades. -

Instructions Calcein AM Cell Viability
IFU0211 Rev 1 Status: RELEASED printed 12/9/2016 11:41:26 AM by Trevigen Document Control Instructions For Research Use Only. Not For Use In Diagnostic Procedures Calcein AM Cell Viability Kit Catalog# 4892-010-K 1000 Tests* *Calculated based on using 1 μM final concentration of Calcein AM; Total number of tests varies with the concentration of Calcein AM required for particular cells. IFU0211 Rev 1 Status: RELEASED printed 12/9/2016 11:41:26 AM by Trevigen Document Control Calcein AM Cell Viability Kit Cat# 4892-010-K Table of Contents Page I. Introduction 1 II. Precautions and Limitations 2 III. Materials Supplied 2 IV. Materials/Equipment Required but not Supplied 2 V. Reagent Preparation 2 VI. Assay Protocol 3 VII. Standardization 4 VIII. Troubleshooting 5 IX. References 5 X. Related Products Available from Trevigen 5 © 2011 Trevigen, Inc. All rights reserved. Trevigen and TACS are registered trademarks and, FlowTACS, TiterTACS, MitoShift and DePsipher are trademarks of Trevigen, Inc. TACS: Trevigen Apoptotic Cell System i E2/25/11v1 IFU0211 Rev 1 Status: RELEASED printed 12/9/2016 11:41:26 AM by Trevigen Document Control I. Introduction Trevigen’s Calcein AM Cell Viability Kit provides a simple, rapid and accurate method to measure cell viability and/or cytotoxicity. Calcein AM (structure A) is a non-fluorescent, hydrophilic compound that easily permeates intact, live cells. The hydrolysis of Calcein AM by intracellular esterases produces calcein (structure B), a hydrophilic, strongly fluorescent compound that is well-retained in the cell cytoplasm. Cells grown in black-walled plates can be stained and quantified in less than two hours. -

Ca2+-Activated Kca3.1 Potassium Channels Contribute to the Slow
www.nature.com/scientificreports OPEN Ca2+‑activated KCa3.1 potassium channels contribute to the slow afterhyperpolarization in L5 neocortical pyramidal neurons M. V. Roshchin, V. N. Ierusalimsky, P. M. Balaban & E. S. Nikitin* Layer 5 neocortical pyramidal neurons are known to display slow Ca2+‑dependent afterhyperpolarization (sAHP) after bursts of spikes, which is similar to the sAHP in CA1 hippocampal cells. However, the mechanisms of sAHP in the neocortex remain poorly understood. Here, we identifed the Ca2+‑gated potassium KCa3.1 channels as contributors to sAHP in ER81‑positive neocortical pyramidal neurons. Moreover, our experiments strongly suggest that the relationship between sAHP and KCa3.1 channels in a feedback mechanism underlies the adaptation of the spiking frequency of layer 5 pyramidal neurons. We demonstrated the relationship between KCa3.1 channels and sAHP using several parallel methods: electrophysiology, pharmacology, immunohistochemistry, and photoactivatable probes. Our experiments demonstrated that ER81 immunofuorescence in layer 5 co‑localized with KCa3.1 immunofuorescence in the soma. Targeted Ca2+ uncaging confrmed two major features of KCa3.1 channels: preferential somatodendritic localization and Ca2+‑driven gating. In addition, both the sAHP and the slow Ca2+‑induced hyperpolarizing current were sensitive to TRAM‑ 34, a selective blocker of KCa3.1 channels. Te neocortex is the largest part of the cortex (~ 90% of the entire cortex in humans), and plays a crucial role in higher functions of the brain such as interpretation of sensory information, formation and storage of long-term memory, language usage, and control of voluntary movements. Pyramidal neurons play a key role in all cortical activities and constitute ~ 80% of all neocortical neurons. -

Advances in Engineering of Fluorescent Proteins And
Author's personal copy Available online at www.sciencedirect.com Advances in engineering of fluorescent proteins and photoactivatable proteins with red emission Kiryl D Piatkevich and Vladislav V Verkhusha Monomeric fluorescent proteins of different colors are widely energy transfer (FRET) approach to three and four colors used to study behavior and targeting of proteins in living cells. in a single cell [3]. Fluorescent proteins that irreversibly change their spectral properties in response to light irradiation of a specific The RFPs, whose chromophores are formed by induction wavelength, or photoactivate, have become increasingly with light, are known as the photoactivatable FPs (PA- popular to image intracellular dynamics and superresolution RFPs). Two different groups of PA-RFPs are presently protein localization. Until recently, however, no optimized being distinguished. Members of the first group exhibit monomeric red fluorescent proteins and red photoactivatable an irreversible photoconversion from the non-fluorescent proteins have been available. Furthermore, monomeric or green fluorescent state to the red fluorescent state. fluorescent proteins, which change emission from blue to red Members of the second group undergo reversible photo- simply with time, so-called fluorescent timers, were developed switching between the non-fluorescent and fluorescent to study protein age and turnover. Understanding of chemical states. Introduction of photoactivatable FPs into cell mechanisms of the chromophore maturation or biology greatly extended the spatio-temporal limits of photoactivation into a red form will further advance engineering in vivo biological dynamics [4] and have become useful of fluorescent timers and photoactivatable proteins with tools for the superresolution microscopy approaches such enhanced and novel properties. -
Calcium Detection Probes & Assay Kits
Calcium Detection Probes & Assay Kits 2016-2017 Cal-520™ Cal-590™ Fluo-8® AAT Bioquest® Advancing Assay & Test Technologies Our Mission AAT Bioquest® is committed to constantly meet or exceed its customer’s requirements by providing consistently high quality products and services, and by encouraging continuous improvements in its long-term and daily operations. Our core value is Innovation and Customer Satisfaction. Our Story AAT Bioquest®, Inc. (formerly ABD Bioquest, Inc.) develops, manufactures and markets bioanalytical research reagents and kits to life sciences research, diagnostic R&D and drug discovery. We specialize in photometric detections including absorption (color), fluorescence and luminescence technologies. The Company's superior products enable life science researchers to better under- stand biochemistry, immunology, cell biology and molecular biology. AAT Bioquest offers a rapidly expanding list of enabling products. Besides the standard catalog products, we also offer custom services to meet the distinct needs of each customer. Our current services include custom synthesis of biological detection probes, custom development of biochemical, cell-based and diagnostic assays and custom high throughput screening of drug discovery targets. It is my greatest pleasure to welcome you to AAT Bioquest. We greatly appreciate the constant support of our valuable customers. While we continue to rapidly expand, our core value remains the same: Innovation and Customer Satisfaction. We are committed to being the leading provider of novel biological detection solutions. We promise to extend these values to you during the course of our service and to continue to support you with our new products and services. It is our greatest honor to receive valuable feedbacks and suggestions from you so that we can better serve your projects. -
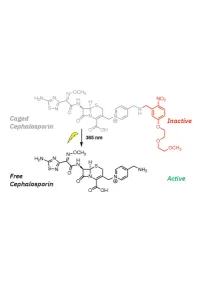
1 20 Introduction
1 Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics 2 3 Inga S. Shchelik, Andrea Tomio, and Karl Gademann 4 Department of Chemistry, University of Zurich, Winterthurerstrasse 190, 8057, Zurich, SwitzerlanD 5 6 7 ABSTRACT 8 The spatial anD temporal control of bioactivity of small molecules by light (photopharmacology) constitutes a 9 promising approach for stuDy of biological processes and ultimately for the treatment of Diseases. In this stuDy, we 10 investigateD two Different ‘cageD’ antibiotic classes that can unDergo remote activation with UV-light at λ=365 nm, 11 via the conjugation of deactivating and photocleavable units through a short synthetic sequence. The two wiDely useD 12 antibiotics vancomycin and cephalosporin were thus enhanceD in their performance by renDering them 13 photoresponsive and thus suppressing undesired off-site activity. The antimicrobial activity against Bacillus subtilis 14 ATCC 6633, Staphylococcus aureus ATCC 29213, S. aureus ATCC 43300 (MRSA), Escherichia coli ATCC 25922, 15 and Pseudomonas aeruginosa ATCC 27853 could be spatiotemporally controlleD with light. Both molecular series 16 displayed a good activity winDow. The vancomycin Derivative DisplayeD excellent values against Gram-positive 17 strains after uncaging, and the next-generation caged cephalosporin derivative achieved good and broad activity 18 against both Gram-positive and Gram-negative strains after photorelease. 19 Key worDs: antibacterial agents, photopharmacology, photocaging, vancomycin, cephalosporin. 1 20 -

Physiological Probes & Assay Kits
Physiological Probes & Assay Kits Calcium Indicators · Membrane Potential Assays · pH Probes ® SIE HABEN DIE VISION, AAT Bioquest WIR HABEN DIE SUBSTANZ. Advancing Assay & Test Technologies www.aatbio.com Labeling Antibodies and Biopolymers Table of Contents Section 1 General Information 2 Section 6 Membrane Potential Measurement 33 Fast Response Membrane Potential Probes ................................................. 35 Section 2 Calcium Ion Detection 5 Slow Response Membrane Potential Probes ............................................... 36 Mitochondrial Membrane Potential Probes ................................................ 37 Fluo-8® Calcium Ion Indicators............................................................... ...... 8 FLIPR® Membrane Potential Assay Kits ....................................................... 38 2 Cal-520™ Calcium Ion Indicators .................................................................10 Rhod-4™ Calcium Ion Indicator ...................................................................11 Section 7 Index 39 Solutions Labeling Fluorescence Optimized BTC Calcium Ion Indicator ...........................................................................12 Fura-2 Calcium Ion Indicator .......................................................................12 Fura-8™ Calcium Ion Indicator ....................................................................12 Alphabetical Index .................................................................................. 40 Indo-1 Calcium Ion Indicator.......................................................................13 -

Regulation of Mitochondrial Permeability Transition Pore by PINK1
Gautier et al. Molecular Neurodegeneration 2012, 7:22 http://www.molecularneurodegeneration.com/content/7/1/22 RESEARCH ARTICLE Open Access Regulation of mitochondrial permeability transition pore by PINK1 Clement A Gautier1,4†, Emilie Giaime1†, Erica Caballero2, Lucía Núñez2, Zhiyin Song3, David Chan3, Carlos Villalobos2 and Jie Shen1* Abstract Background: Loss-of-function mutations in PTEN-induced kinase 1 (PINK1) have been linked to familial Parkinson’s disease, but the underlying pathogenic mechanism remains unclear. We previously reported that loss of PINK1 impairs mitochondrial respiratory activity in mouse brains. Results: In this study, we investigate how loss of PINK1 impairs mitochondrial respiration using cultured primary fibroblasts and neurons. We found that intact mitochondria in PINK1−/− cells recapitulate the respiratory defect in isolated mitochondria from PINK1−/− mouse brains, suggesting that these PINK1−/− cells are a valid experimental system to study the underlying mechanisms. Enzymatic activities of the electron transport system complexes are normal in PINK1−/− cells, but mitochondrial transmembrane potential is reduced. Interestingly, the opening of the mitochondrial permeability transition pore (mPTP) is increased in PINK1−/− cells, and this genotypic difference between PINK1−/− and control cells is eliminated by agonists or inhibitors of the mPTP. Furthermore, inhibition of mPTP opening rescues the defects in transmembrane potential and respiration in PINK1−/− cells. Consistent with our earlier findings in mouse brains, mitochondrial morphology is similar between PINK1−/− and wild-type cells, indicating that the observed mitochondrial functional defects are not due to morphological changes. Following FCCP treatment, calcium increases in the cytosol are higher in PINK1−/− compared to wild-type cells, suggesting that intra-mitochondrial calcium concentration is higher in the absence of PINK1. -

Abstract 30-Day Immunotoxicity Study of PFMOAA in C57BL/6 Mice
Abstract 30-Day Immunotoxicity Study of PFMOAA in C57BL/6 Mice by Samuel Vance July, 2019 Director of Thesis: Dr. Jamie DeWitt, Department of Pharmacology and Toxicology Within the past five years, two classes of per- and polyfluoroalkyl substances (PFAS) were phased out of production in the U.S., which led to the development and production of PFAS to replace these two major classes. One family of these PFAS are perfluoro-ether carboxylic acids (PFECA), which have emerged in the public and scientific arenas due to their presence in drinking water systems across the U.S., including Wilmington, NC. Although manufacturers have touted them as having more favorable environmental and toxicological properties very little is known about the toxicity and environmental fate these emerging PFECA. One compound, perfluoro-2-methoxyacetic acid (PFMOAA), was identified as the dominant PFECA in the Cape Fear River, in concentrations as high as 35,000 ng/L. There is very little mention of PFMOAA in the publicly available scientific literature and to our knowledge, we are the first to investigate its potential for toxic effects. In this 30-day study, we orally administered 25,000, 2,500,000, or 250,000,000 ng/L of PFMOAA in water to male and female C57BL/6 mice and investigated immune and liver alterations following exposure. Mice given PFMOAA showed no signs of overt toxicity during the study and no evident changes were observed in liver mass or peroxisomal enzyme activity. While mild alterations in splenic and thymic lymphocyte sub- populations were observed in males, these results do not point to any definitive alterations in immune function.