Rational Design of Small Molecule Fluorescent Probes for Biological Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Single-Molecule Spectroscopy and Imaging of Biomolecules in Living Cells
Anal. Chem. 2010, 82, 2192–2203 Single-Molecule Spectroscopy and Imaging of Biomolecules in Living Cells Samuel J. Lord, Hsiao-lu D. Lee, and W. E. Moerner* Department of Chemistry, Stanford University, Stanford, California 94305-5080 The number of reports per year on single-molecule imaging instance, the shape of the distribution may be skewed or reveal experiments has grown roughly exponentially since the first multiple subpopulations, which may offer insight into underlying successful efforts to optically detect a single molecule were mechanisms. Each single molecule is a local reporter on the completed over two decades ago. Single-molecule spectros- makeup and conditions of its immediate surroundings, its “na- copy has developed into a field that includes a wealth of noenvironment”, and thus acts as a readout of spatial heterogene- experiments at room temperature and inside living cells. The ity of a sample. SMS also measures time-dependent processes that fast growth of single-molecule biophysics has resulted from are not necessarily synchronized throughout the sample or its benefits in probing heterogeneous populations, one population. For example, multiple catalytic states of an enzyme molecule at a time, as well as from advances in microscopes will be convolved with all the states of other copies in an ensemble, and detectors. This Perspective summarizes the field of live- whereas a SMS experiment can measure uncorrelated stochastic cell imaging of single biomolecules. transitions of a single enzyme. SMS also has the ability to observe intermediate states or rare events, given that the instruments have Single-molecule biophysics spans a range of experiments, from sufficient time resolution. -

Topological Model on the Inductive Effect in Alkyl Halides Using Local Quantum Similarity and Reactivity Descriptors in the Density Functional Theory
Hindawi Publishing Corporation Journal of Quantum Chemistry Volume 2014, Article ID 850163, 12 pages http://dx.doi.org/10.1155/2014/850163 Research Article Topological Model on the Inductive Effect in Alkyl Halides Using Local Quantum Similarity and Reactivity Descriptors in the Density Functional Theory Alejandro Morales-Bayuelo1,2 and Ricardo Vivas-Reyes1 1 Grupo de Qu´ımica Cuantica´ y Teorica,´ Universidad de Cartagena, Programa de Qu´ımica, Facultad de Ciencias Exactas y Naturales, Cartagena de Indias, Colombia 2 Departamento de Ciencias Qu´ımicas, Universidad Nacional Andres Bello, Republica 275, Santiago, Chile Correspondence should be addressed to Alejandro Morales-Bayuelo; [email protected] and Ricardo Vivas-Reyes; [email protected] Received 19 September 2013; Revised 16 December 2013; Accepted 16 December 2013; Published 19 February 2014 Academic Editor: Daniel Glossman-Mitnik Copyright © 2014 A. Morales-Bayuelo and R. Vivas-Reyes. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We present a topological analysis to the inductive effect through steric and electrostatic scales of quantitative convergence. Using the molecular similarity field based in the local guantum similarity (LQS) with the Topo-Geometrical Superposition Algorithm (TGSA) alignment method and the chemical reactivity in the density function theory (DFT) context, all calculations were carried out with Amsterdam Density Functional (ADF) code, using the gradient generalized approximation (GGA) and local exchange correlations PW91, in order to characterize the electronic effect by atomic size in the halogens group using a standard Slater-type- orbital basis set. -

Understanding Nonplanarity in Metallabenzene Complexes
1986 Organometallics 2007, 26, 1986-1995 Understanding Nonplanarity in Metallabenzene Complexes Jun Zhu, Guochen Jia,* and Zhenyang Lin* Department of Chemistry, The Hong Kong UniVersity of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, People’s Republic of China ReceiVed February 11, 2007 The nonplanarity found in metallabenzene complexes has been investigated theoretically via density functional theory (DFT) calculations. A metallabenzene has four occupied π molecular orbitals (8 π electrons) instead of three that benzene has. Our electronic structure analyses show that the extra occupied π molecular orbital, which is the highest occupied molecular orbital (HOMO) in many metallabenzenes, has antibonding interactions between the metal center and the metal-bonded ring-carbon atoms, providing the electronic driving force toward nonplanarity. Calculations indicate that the electronic driving force toward nonplanarity, however, is relatively small. Therefore, other factors such as steric effects also play important roles in determining the planarity of these metallabenzene complexes. In this paper, how the various electronic and steric factors interplay has been discussed. Introduction benzene complexes. For example, the formation mechanism and chemical reactivity of metallabenzene complexes have been Metallabenzenes, organometallic compounds formed by extensively studied.17 formal replacement of a CH group in benzene by an isolobal In the studies of metallabenzene complexes, a central issue transition metal fragment, were first considered theoretically by concerns the π-conjugation of the six-membered metal-contain- Hoffman et al. in 1979.1 Since the isolation of the first stable 2 ing ring. Indeed, it is true that metallabenzene complexes are osmabenzenes by Roper’s group in 1982, metallabenzene highly conjugated in view of the fact that the single-double complexes have attracted considerable interest over the last bond alternation is insignificant in the six-membered metal- quarter century. -

Product Information Sheet
AAT Bioquest®, Inc. Product Technical Information Sheet Last Updated July 2012 Classic Calcium Detection Reagents Calcium acts as a universal second messenger in a variety of cells. Numerous functions of all types of cells are regulated by Ca2+ to a greater or lesser degree, thus calcium measurement is critical for numerous biological investigations. Since the 1920s, scientists have attempted to measure Ca2+, but few were successful due to limited availability of Ca2+ probes. The first reliable measurement of Ca2+ was performed by Ridgway and Ashley by injecting the photoprotein aequorin into the giant muscle fiber of the barnacle. Subsequently, in the 1980s, Tsien and colleagues produced a variety of fluorescent indicators. Among them Indo-1, Fura-2, Fluo-3 and Rhod-2 have been the most valuable dyes for measuring Ca2+ with a fluorescence instrument. Fluorescent probes that show spectral responses upon binding to Ca2+ have enabled researchers to investigate changes in intracellular free Ca2+ concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. Most of these fluorescent indicators are derivatives of BAPTA chelators that incorporate a PET system responsive to calcium. FLIPR® and FlexStation™ instruments of Molecular Devices Corp., FDSS of Hamamatsu Corp. and NOVOstar™ of BMG Technologies have enabled high throughput measurement of calcium for GPCR and ion channel research. There are quite a few factors that need to be considered when selecting a fluorescent Ca2+ indicator. Spectral Properties: For UV excitation, Indo-1 and Fura-2 are widely used. Fluo-3 is preferred for 488 nm excitation while Rhod-2 and X-rhod are used for red emissions. -

Recent Advances in Chemical Biology Using Benzophenones and Diazirines As Radical Precursors
molecules Review Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors 1,2, , 1,2, Muhammad Murtaza Hassan * y and Olasunkanmi O. Olaoye y 1 Department of Chemical and Physical Sciences, University of Toronto Mississauga, 3359 Mississauga Road North, Mississauga, ON L5L 1C6, Canada; [email protected] 2 Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON M5S 3H6, Canada * Correspondence: [email protected]; Tel.: +1-905-569-4588 These authors contributed equally to this work. y Academic Editor: Edward Lee-Ruff Received: 18 April 2020; Accepted: 9 May 2020; Published: 13 May 2020 Abstract: The use of light-activated chemical probes to study biological interactions was first discovered in the 1960s, and has since found many applications in studying diseases and gaining deeper insight into various cellular mechanisms involving protein–protein, protein–nucleic acid, protein–ligand (drug, probe), and protein–co-factor interactions, among others. This technique, often referred to as photoaffinity labelling, uses radical precursors that react almost instantaneously to yield spatial and temporal information about the nature of the interaction and the interacting partner(s). This review focuses on the recent advances in chemical biology in the use of benzophenones and diazirines, two of the most commonly known light-activatable radical precursors, with a focus on the last three years, and is intended to provide a solid understanding of their chemical and biological principles and their applications. Keywords: photoaffinity labelling; benzophenone; diazirine; radical precursors; interactome; SABRE; hyperpolarizing agents; crosslinking; photochemistry 1. Introduction The use of radicals or photoactivatable radical precursors has become ubiquitous in the fields of medicinal chemistry and chemical biology in the past three decades. -

Principles of Drug Action 1, Spring 2005, Resonance and Induction
Principles Of Drug Action 1, Spring 2005, Resonance and Induction RESONANCE AND INDUCTION TUTORIAL Jack DeRuiter The terms "resonance" and "induction" refer to the electronic effects that atoms or functional groups may have within a compound. These effects are defined below and are dependent on the valence, bonding order and electronegativity of atoms, as well as the molecular geometry. Thus it is important that you understand these concepts prior to reviewing this tutorial. I. INDUCTION Induction or the inductive effect of an atom or functional group is a function of that groups 1). electronegativity, 2). bonding order and charge and 3). position within a structure. Inductive effects refer to those electronic effects of an atom or functional group can contribute through single bonds such as saturated (sp3) carbon atoms! This is very different from resonance effects discussed later in this section. The contribution of electronegativity, bonding order and position toward induction is as follows: Electronegativity: Atoms or functional groups that are electronegative relative to hydrogen such as the halogens, oxygen, nitrogen, etc. may have a negative inductive effect (-I), depending on their bonding order (see the Table below). Thus these atoms withdraw electron density through the single bond structure of a compound and can assist in the stabilization of negative charge that may form in reactions. One such reaction where -I groups can have a stabilizing (enhancing) effect is the ionization of acids. Consider the case of acetic acid, chloroacetic acid and trichloroacetic acid shown below. All three of these compounds can ionize (loss of proton from the carboxyl OH). -

Ca2+-Activated Kca3.1 Potassium Channels Contribute to the Slow
www.nature.com/scientificreports OPEN Ca2+‑activated KCa3.1 potassium channels contribute to the slow afterhyperpolarization in L5 neocortical pyramidal neurons M. V. Roshchin, V. N. Ierusalimsky, P. M. Balaban & E. S. Nikitin* Layer 5 neocortical pyramidal neurons are known to display slow Ca2+‑dependent afterhyperpolarization (sAHP) after bursts of spikes, which is similar to the sAHP in CA1 hippocampal cells. However, the mechanisms of sAHP in the neocortex remain poorly understood. Here, we identifed the Ca2+‑gated potassium KCa3.1 channels as contributors to sAHP in ER81‑positive neocortical pyramidal neurons. Moreover, our experiments strongly suggest that the relationship between sAHP and KCa3.1 channels in a feedback mechanism underlies the adaptation of the spiking frequency of layer 5 pyramidal neurons. We demonstrated the relationship between KCa3.1 channels and sAHP using several parallel methods: electrophysiology, pharmacology, immunohistochemistry, and photoactivatable probes. Our experiments demonstrated that ER81 immunofuorescence in layer 5 co‑localized with KCa3.1 immunofuorescence in the soma. Targeted Ca2+ uncaging confrmed two major features of KCa3.1 channels: preferential somatodendritic localization and Ca2+‑driven gating. In addition, both the sAHP and the slow Ca2+‑induced hyperpolarizing current were sensitive to TRAM‑ 34, a selective blocker of KCa3.1 channels. Te neocortex is the largest part of the cortex (~ 90% of the entire cortex in humans), and plays a crucial role in higher functions of the brain such as interpretation of sensory information, formation and storage of long-term memory, language usage, and control of voluntary movements. Pyramidal neurons play a key role in all cortical activities and constitute ~ 80% of all neocortical neurons. -

Advances in Engineering of Fluorescent Proteins And
Author's personal copy Available online at www.sciencedirect.com Advances in engineering of fluorescent proteins and photoactivatable proteins with red emission Kiryl D Piatkevich and Vladislav V Verkhusha Monomeric fluorescent proteins of different colors are widely energy transfer (FRET) approach to three and four colors used to study behavior and targeting of proteins in living cells. in a single cell [3]. Fluorescent proteins that irreversibly change their spectral properties in response to light irradiation of a specific The RFPs, whose chromophores are formed by induction wavelength, or photoactivate, have become increasingly with light, are known as the photoactivatable FPs (PA- popular to image intracellular dynamics and superresolution RFPs). Two different groups of PA-RFPs are presently protein localization. Until recently, however, no optimized being distinguished. Members of the first group exhibit monomeric red fluorescent proteins and red photoactivatable an irreversible photoconversion from the non-fluorescent proteins have been available. Furthermore, monomeric or green fluorescent state to the red fluorescent state. fluorescent proteins, which change emission from blue to red Members of the second group undergo reversible photo- simply with time, so-called fluorescent timers, were developed switching between the non-fluorescent and fluorescent to study protein age and turnover. Understanding of chemical states. Introduction of photoactivatable FPs into cell mechanisms of the chromophore maturation or biology greatly extended the spatio-temporal limits of photoactivation into a red form will further advance engineering in vivo biological dynamics [4] and have become useful of fluorescent timers and photoactivatable proteins with tools for the superresolution microscopy approaches such enhanced and novel properties. -

Interpretation of Both Electron Pushing and Electron Withdrawing Inductive Effect of Alkyl Groups in Terms of Mulliken–Jaffe’S Charge Coefficient Parameters (B)∗
GENERAL ARTICLE Interpretation of Both Electron Pushing and Electron Withdrawing Inductive Effect of Alkyl Groups in Terms of Mulliken–Jaffe’s Charge Coefficient Parameters (b)∗ Asim K Das Alkyl groups can delocalize both the negative and positive charges through ion (charge)-induced dipole interaction with the polarizable alkyl groups. This charge induced polariza- tion of the alkyl group to delocalize the charge is the origin of ‘inductive effect’ of the alkyl groups. The apparent less effi- ciency in negative charge delocalization compared to positive charge delocalization by a particular alkyl group is attributed Asim K Das is currently a to the less polarizing field strength of the anionic charge. senior Professor of Chemistry Department, Visva Bharati, a Central University, 1. Introduction Santiniketan. His research interest is in the field of ff thermodynamic and kinetic The concept of inductive e ect and its application are the impor- aspects of metal-ligand tant course components of organic chemistry taught at the high interactions. He has authored school and undergraduate level. Inductive effect actually delocal- some advanced level text izes the charge [1, 2]. Depending on the properties of the atoms books on inorganic chemistry, ff bioinorganic chemistry and or groups, they can show electron withdrawing (i.e. –I e ect) or environmental chemistry for electron pushing (i.e. +Ieffect) inductive effect [1, 2]. The +Ief- the undergraduate and fect delocalizes the positive charge while the −Ieffect delocalizes postgraduate students. the negative charge. By considering the inductive effect of the groups or atoms, many important aspects of organic chemistry like the relative stabilities of carbocations and carbanions, acid- base strength, reaction mechanism, etc., are explained [1, 2] in the classrooms, and these are also discussed in all organic textbooks. -
Calcium Detection Probes & Assay Kits
Calcium Detection Probes & Assay Kits 2016-2017 Cal-520™ Cal-590™ Fluo-8® AAT Bioquest® Advancing Assay & Test Technologies Our Mission AAT Bioquest® is committed to constantly meet or exceed its customer’s requirements by providing consistently high quality products and services, and by encouraging continuous improvements in its long-term and daily operations. Our core value is Innovation and Customer Satisfaction. Our Story AAT Bioquest®, Inc. (formerly ABD Bioquest, Inc.) develops, manufactures and markets bioanalytical research reagents and kits to life sciences research, diagnostic R&D and drug discovery. We specialize in photometric detections including absorption (color), fluorescence and luminescence technologies. The Company's superior products enable life science researchers to better under- stand biochemistry, immunology, cell biology and molecular biology. AAT Bioquest offers a rapidly expanding list of enabling products. Besides the standard catalog products, we also offer custom services to meet the distinct needs of each customer. Our current services include custom synthesis of biological detection probes, custom development of biochemical, cell-based and diagnostic assays and custom high throughput screening of drug discovery targets. It is my greatest pleasure to welcome you to AAT Bioquest. We greatly appreciate the constant support of our valuable customers. While we continue to rapidly expand, our core value remains the same: Innovation and Customer Satisfaction. We are committed to being the leading provider of novel biological detection solutions. We promise to extend these values to you during the course of our service and to continue to support you with our new products and services. It is our greatest honor to receive valuable feedbacks and suggestions from you so that we can better serve your projects. -
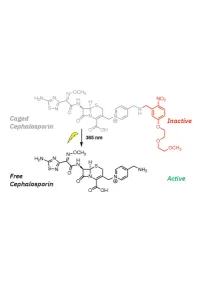
1 20 Introduction
1 Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics 2 3 Inga S. Shchelik, Andrea Tomio, and Karl Gademann 4 Department of Chemistry, University of Zurich, Winterthurerstrasse 190, 8057, Zurich, SwitzerlanD 5 6 7 ABSTRACT 8 The spatial anD temporal control of bioactivity of small molecules by light (photopharmacology) constitutes a 9 promising approach for stuDy of biological processes and ultimately for the treatment of Diseases. In this stuDy, we 10 investigateD two Different ‘cageD’ antibiotic classes that can unDergo remote activation with UV-light at λ=365 nm, 11 via the conjugation of deactivating and photocleavable units through a short synthetic sequence. The two wiDely useD 12 antibiotics vancomycin and cephalosporin were thus enhanceD in their performance by renDering them 13 photoresponsive and thus suppressing undesired off-site activity. The antimicrobial activity against Bacillus subtilis 14 ATCC 6633, Staphylococcus aureus ATCC 29213, S. aureus ATCC 43300 (MRSA), Escherichia coli ATCC 25922, 15 and Pseudomonas aeruginosa ATCC 27853 could be spatiotemporally controlleD with light. Both molecular series 16 displayed a good activity winDow. The vancomycin Derivative DisplayeD excellent values against Gram-positive 17 strains after uncaging, and the next-generation caged cephalosporin derivative achieved good and broad activity 18 against both Gram-positive and Gram-negative strains after photorelease. 19 Key worDs: antibacterial agents, photopharmacology, photocaging, vancomycin, cephalosporin. 1 20 -

Measuring the Electronic Effect of Some Phosphines and Phosphites
Measuring the electronic effect of some phosphines and phosphites By Sanaa Moftah Ali Abushhewa A dissertation submitted to the University of Manchester for the degree of Master of Science by research in the Faculty of Engineering and Physical Sciences. School of Chemistry 2010 Contents page Abstract 6 Declaration 7 Acknowledgement 8 1. Introduction 9 1.1 History of Phosphorus 9 1.2 Phosphorus (III) compounds 10 1.3 Preparation and Properties of Phosphines 11 1.4 Preparation and Properties of Phosphites 12 1.5 Bonding of phosphorus (III) ligands 14 1.6 Measurement of Electronic and Steric Effects 15 1.7 Steric parameter 17 1.8 Electronic parameter 17 1.9 Effects of electronegative substituents 21 1.10 Phosphines and Phosphites ligand effects 21 2. Experimental section 24 General conditions 25 2.1- Preparation of phosphites 25 2.1.1- Synthesis of 2, 2’- Biphenyl Chlorophosphite 25 2.1.2- Synthesis of 1H,1H,2H,2H-perfluorodecyldiphenyl phosphinite 25 2 2.1.3- Synthesis of 3-fluorophenyldiphenyl phosphinite 26 2.1.4- Synthesis of 2-fluorophenyldiphenyl phosphinite 26 2.1.5- Synthesis of 2, 2-biphenyltrifluoroethyl phosphinite 27 2.1.6- Synthesis of 2, 2'-biphenylhexafluoroisopropyl phosphinite 27 2.1.7-Synthesis of 2, 2'-biphenylpropyl phosphite 28 2.2-Preparation of P(III) Selenides 28 2.2.1- Synthesis of triphenylphosphine selenide 28 2.2.2- Synthesis of triphenylphosphite selenide 29 2.2.3- Synthesis of tris(2,4,6-trimethylphenyl)phosphite selenide 29 2.2.4- Synthesis of diphenyl(o-tolyl) phosphine selenide 30 2.2.5- Synthesis of 2, 2’-biphenyl