Ca2+-Activated Kca3.1 Potassium Channels Contribute to the Slow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Single-Molecule Spectroscopy and Imaging of Biomolecules in Living Cells
Anal. Chem. 2010, 82, 2192–2203 Single-Molecule Spectroscopy and Imaging of Biomolecules in Living Cells Samuel J. Lord, Hsiao-lu D. Lee, and W. E. Moerner* Department of Chemistry, Stanford University, Stanford, California 94305-5080 The number of reports per year on single-molecule imaging instance, the shape of the distribution may be skewed or reveal experiments has grown roughly exponentially since the first multiple subpopulations, which may offer insight into underlying successful efforts to optically detect a single molecule were mechanisms. Each single molecule is a local reporter on the completed over two decades ago. Single-molecule spectros- makeup and conditions of its immediate surroundings, its “na- copy has developed into a field that includes a wealth of noenvironment”, and thus acts as a readout of spatial heterogene- experiments at room temperature and inside living cells. The ity of a sample. SMS also measures time-dependent processes that fast growth of single-molecule biophysics has resulted from are not necessarily synchronized throughout the sample or its benefits in probing heterogeneous populations, one population. For example, multiple catalytic states of an enzyme molecule at a time, as well as from advances in microscopes will be convolved with all the states of other copies in an ensemble, and detectors. This Perspective summarizes the field of live- whereas a SMS experiment can measure uncorrelated stochastic cell imaging of single biomolecules. transitions of a single enzyme. SMS also has the ability to observe intermediate states or rare events, given that the instruments have Single-molecule biophysics spans a range of experiments, from sufficient time resolution. -

Membrane Properties Underlying the Firing of Neurons in the Avian Cochlear Nucleus
The Journal of Neuroscience, September 1994, 74(g): 53526364 Membrane Properties Underlying the Firing of Neurons in the Avian Cochlear Nucleus A. D. Reyes,i*2 E. W Rubel,* and W. J. Spain2,3z4 ‘Department of Medical Genetics, *Virginia Merrill Bloedel Hearing Research Center, and 3Department of Medicine (Division of Neurology), University of Washington School of Medicine, Seattle, Washington 98195, and 4Seattle VA Medical Center, Seattle, Washington 98108 Neurons of the avian nucleus magnocellularis (NM) relay The avian nucleus magnocellularis(NM) contains the second- auditory information from the Vlllth nerve to other parts of order auditory neurons that serve as a relay in the pathway the auditory system. To examine the cellular properties that responsiblefor sound localization in the azimuth. NM neurons permit NM neurons to transmit reliably the temporal char- are innervated by VIIIth nerve fibers and project bilaterally to acteristics of the acoustic stimulus, we performed whole- neurons of the nucleus laminaris (NL, Parks and Rubel, 1975; cell recordings in neurons of the chick NM using an in vitro Rubel and Parks, 1975; Young and Rubel, 1983; Carr and Kon- thin slice preparation. NM neurons exhibited strong outward ishi, 1990). Differences in the times of arrival of sound to the rectification near resting potential; the voltage responses to two ears, which vary with location of sound, translate to dif- depolarizing current steps were substantially smaller than ferencesin the arrival times of impulses to NL from the ipsi- to equivalent hyperpolarizing steps. Suprathreshold current lateral and contralateral NM (Overholt et al., 1992; Josephand steps evoked only a single action potential at the start of Hyson, 1993). -

Rational Design of Small Molecule Fluorescent Probes for Biological Applications
Organic & Biomolecular Chemistry Rational Design of Small Molecule Fluorescent Probes for Biological Applications Journal: Organic & Biomolecular Chemistry Manuscript ID OB-REV-06-2020-001131.R1 Article Type: Review Article Date Submitted by the 13-Jul-2020 Author: Complete List of Authors: Jun, Joomyung; University of Pennsylvania, Chemistry; Massachusetts Institute of Technology, Chemistry Chenoweth, David; University of Pennsylvania, Department of Chemistry Petersson, E.; University of Pennsylvania, Chemistry Page 1 of 16 Organic & Biomolecular Chemistry ARTICLE Rational Design of Small Molecule Fluorescent Probes for Biological Applications a,b a a,c Received 00th January 20xx, Joomyung V. Jun, David M. Chenoweth* and E. James Petersson* Accepted 00th January 20xx Fluorescent small molecules are powerful tools for visualizing biological events, embodying an essential facet of chemical DOI: 10.1039/x0xx00000x biology. Since the discovery of the first organic fluorophore, quinine, in 1845, both synthetic and theoretical efforts have endeavored to “modulate” fluorescent compounds. An advantage of synthetic dyes is the ability to employ modern organic chemistry strategies to tailor chemical structures and thereby rationally tune photophysical properties and functionality of the fluorophore. This review explores general factors affecting fluorophore excitation and emission spectra, molar absorption, Stokes shift, and quantum efficiency; and provides guidelines for chemist to create novel probes. Structure- property relationships concerning the substituents are discussed in detail with examples for several dye families. Then, we present a survey of functional probes based on PeT, FRET, and environmental or photo-sensitivity, focusing on representative recent work in each category. We believe that a full understanding of dyes with diverse chemical moieties enables the rational design of probes for the precise interrogation of biochemical and biological phenomena. -

Product Information Sheet
AAT Bioquest®, Inc. Product Technical Information Sheet Last Updated July 2012 Classic Calcium Detection Reagents Calcium acts as a universal second messenger in a variety of cells. Numerous functions of all types of cells are regulated by Ca2+ to a greater or lesser degree, thus calcium measurement is critical for numerous biological investigations. Since the 1920s, scientists have attempted to measure Ca2+, but few were successful due to limited availability of Ca2+ probes. The first reliable measurement of Ca2+ was performed by Ridgway and Ashley by injecting the photoprotein aequorin into the giant muscle fiber of the barnacle. Subsequently, in the 1980s, Tsien and colleagues produced a variety of fluorescent indicators. Among them Indo-1, Fura-2, Fluo-3 and Rhod-2 have been the most valuable dyes for measuring Ca2+ with a fluorescence instrument. Fluorescent probes that show spectral responses upon binding to Ca2+ have enabled researchers to investigate changes in intracellular free Ca2+ concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. Most of these fluorescent indicators are derivatives of BAPTA chelators that incorporate a PET system responsive to calcium. FLIPR® and FlexStation™ instruments of Molecular Devices Corp., FDSS of Hamamatsu Corp. and NOVOstar™ of BMG Technologies have enabled high throughput measurement of calcium for GPCR and ion channel research. There are quite a few factors that need to be considered when selecting a fluorescent Ca2+ indicator. Spectral Properties: For UV excitation, Indo-1 and Fura-2 are widely used. Fluo-3 is preferred for 488 nm excitation while Rhod-2 and X-rhod are used for red emissions. -

Recent Advances in Chemical Biology Using Benzophenones and Diazirines As Radical Precursors
molecules Review Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors 1,2, , 1,2, Muhammad Murtaza Hassan * y and Olasunkanmi O. Olaoye y 1 Department of Chemical and Physical Sciences, University of Toronto Mississauga, 3359 Mississauga Road North, Mississauga, ON L5L 1C6, Canada; [email protected] 2 Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON M5S 3H6, Canada * Correspondence: [email protected]; Tel.: +1-905-569-4588 These authors contributed equally to this work. y Academic Editor: Edward Lee-Ruff Received: 18 April 2020; Accepted: 9 May 2020; Published: 13 May 2020 Abstract: The use of light-activated chemical probes to study biological interactions was first discovered in the 1960s, and has since found many applications in studying diseases and gaining deeper insight into various cellular mechanisms involving protein–protein, protein–nucleic acid, protein–ligand (drug, probe), and protein–co-factor interactions, among others. This technique, often referred to as photoaffinity labelling, uses radical precursors that react almost instantaneously to yield spatial and temporal information about the nature of the interaction and the interacting partner(s). This review focuses on the recent advances in chemical biology in the use of benzophenones and diazirines, two of the most commonly known light-activatable radical precursors, with a focus on the last three years, and is intended to provide a solid understanding of their chemical and biological principles and their applications. Keywords: photoaffinity labelling; benzophenone; diazirine; radical precursors; interactome; SABRE; hyperpolarizing agents; crosslinking; photochemistry 1. Introduction The use of radicals or photoactivatable radical precursors has become ubiquitous in the fields of medicinal chemistry and chemical biology in the past three decades. -

ELECTROPHYSIOLOGY of the NEURON an Interactive Tutorial
ELECTROPHYSIOLOGY OF THE NEURON An Interactive Tutorial JOHN HUGUENARD DAVID A. MCCORMICK A Companion to Neurobiology by Gordon Shepherd New York Oxford OXFORD UNIVERSITY PRESS 1994 Oxford University Press Oxford New York Toronto Delhi Bombay Calcutta Madras Karachi Kuala Lumpur Singapore Hong Kong Tokyo Nairobi Dar es Salaam Cape Town Melbourne Auckland Madrid and associated companies in Berlin Ibadan Copyright © 1994 by Oxford University Press, Inc. Published by Oxford University Press, Inc., 198 Madison Avenue, New York, New York 10016-4314 Oxford is a registered trademark of Oxford University Press All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of Oxford University Press. Library of Congress Cataloging-in-Publication Data Huguenard, John. Electrophysiolqgy of the neuron : an interactive tutorial / John Huguenard and David A. McCormick. p. cm. Companion to: Neurobiology by Gordon Shepherd. Includes bibliographical references. ISBN 0-19-509167-1 1. Neurophysiology—Computer simulation—Laboratory manuals. 2. Neurons—Computer simulation—Laboratory manuals. 3. Electrophysiology—Computer simulation—Laboratory manuals. I. McCormick, David. II. Shepherd, Gordon M., 1933—Neurobiology. in. Title. QP357.H84 1994 612.8—dc20 94-6530 35798642 Printed in the United States of America on acid-free paper Contents Introduction 3 Installation 4 Using a Computer to Perform -

Advances in Engineering of Fluorescent Proteins And
Author's personal copy Available online at www.sciencedirect.com Advances in engineering of fluorescent proteins and photoactivatable proteins with red emission Kiryl D Piatkevich and Vladislav V Verkhusha Monomeric fluorescent proteins of different colors are widely energy transfer (FRET) approach to three and four colors used to study behavior and targeting of proteins in living cells. in a single cell [3]. Fluorescent proteins that irreversibly change their spectral properties in response to light irradiation of a specific The RFPs, whose chromophores are formed by induction wavelength, or photoactivate, have become increasingly with light, are known as the photoactivatable FPs (PA- popular to image intracellular dynamics and superresolution RFPs). Two different groups of PA-RFPs are presently protein localization. Until recently, however, no optimized being distinguished. Members of the first group exhibit monomeric red fluorescent proteins and red photoactivatable an irreversible photoconversion from the non-fluorescent proteins have been available. Furthermore, monomeric or green fluorescent state to the red fluorescent state. fluorescent proteins, which change emission from blue to red Members of the second group undergo reversible photo- simply with time, so-called fluorescent timers, were developed switching between the non-fluorescent and fluorescent to study protein age and turnover. Understanding of chemical states. Introduction of photoactivatable FPs into cell mechanisms of the chromophore maturation or biology greatly extended the spatio-temporal limits of photoactivation into a red form will further advance engineering in vivo biological dynamics [4] and have become useful of fluorescent timers and photoactivatable proteins with tools for the superresolution microscopy approaches such enhanced and novel properties. -

A Neurophysiologically-Inspired Statistical Language Model
A Neurophysiologically-Inspired Statistical Language Model Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jon Dehdari ∼6 6 Graduate Program in Linguistics The Ohio State University 2014 Dissertation Committee: Professor William Schuler, Advisor Professor Eric Fosler-Lussier Professor Per Sederberg c Jon Dehdari, 2014 Abstract We describe a statistical language model having components that are inspired by electrophysiological activities in the brain. These components correspond to important language-relevant event-related potentials measured using electroencephalography. We relate neural signals involved in local- and long-distance grammatical processing, as well as local- and long-distance lexical processing to statistical language models that are scalable, cross-linguistic, and incremental. We develop a novel language model component that unifies n-gram, skip, and trigger language models into a generalized model inspired by the long-distance lexical event-related potential (N400). We evaluate this model in textual and speech recognition experiments, showing consistent improvements over 4-gram modified Kneser-Ney language models (Chen and Goodman, 1998) for large-scale textual datasets in English, Arabic, Croatian, and Hungarian. ii Acknowledgements I would like to thank my advisor, William Schuler, for his guidance these past few years. His easygoing attitude combined with his passion for his research interests helped steer me towards the topic of this dissertation. I'm also grateful for helpful feedback from Eric Fosler-Lussier and Per Sederberg in improving this work. Eric also provided much-appreciated guidance during my candidacy exams. I'd like to thank my past advisors, Chris Brew, Detmar Meurers, and Deryle Lonsdale for shaping who I am today, and for their encouragement and instruction. -
Calcium Detection Probes & Assay Kits
Calcium Detection Probes & Assay Kits 2016-2017 Cal-520™ Cal-590™ Fluo-8® AAT Bioquest® Advancing Assay & Test Technologies Our Mission AAT Bioquest® is committed to constantly meet or exceed its customer’s requirements by providing consistently high quality products and services, and by encouraging continuous improvements in its long-term and daily operations. Our core value is Innovation and Customer Satisfaction. Our Story AAT Bioquest®, Inc. (formerly ABD Bioquest, Inc.) develops, manufactures and markets bioanalytical research reagents and kits to life sciences research, diagnostic R&D and drug discovery. We specialize in photometric detections including absorption (color), fluorescence and luminescence technologies. The Company's superior products enable life science researchers to better under- stand biochemistry, immunology, cell biology and molecular biology. AAT Bioquest offers a rapidly expanding list of enabling products. Besides the standard catalog products, we also offer custom services to meet the distinct needs of each customer. Our current services include custom synthesis of biological detection probes, custom development of biochemical, cell-based and diagnostic assays and custom high throughput screening of drug discovery targets. It is my greatest pleasure to welcome you to AAT Bioquest. We greatly appreciate the constant support of our valuable customers. While we continue to rapidly expand, our core value remains the same: Innovation and Customer Satisfaction. We are committed to being the leading provider of novel biological detection solutions. We promise to extend these values to you during the course of our service and to continue to support you with our new products and services. It is our greatest honor to receive valuable feedbacks and suggestions from you so that we can better serve your projects. -
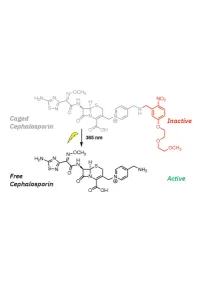
1 20 Introduction
1 Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics 2 3 Inga S. Shchelik, Andrea Tomio, and Karl Gademann 4 Department of Chemistry, University of Zurich, Winterthurerstrasse 190, 8057, Zurich, SwitzerlanD 5 6 7 ABSTRACT 8 The spatial anD temporal control of bioactivity of small molecules by light (photopharmacology) constitutes a 9 promising approach for stuDy of biological processes and ultimately for the treatment of Diseases. In this stuDy, we 10 investigateD two Different ‘cageD’ antibiotic classes that can unDergo remote activation with UV-light at λ=365 nm, 11 via the conjugation of deactivating and photocleavable units through a short synthetic sequence. The two wiDely useD 12 antibiotics vancomycin and cephalosporin were thus enhanceD in their performance by renDering them 13 photoresponsive and thus suppressing undesired off-site activity. The antimicrobial activity against Bacillus subtilis 14 ATCC 6633, Staphylococcus aureus ATCC 29213, S. aureus ATCC 43300 (MRSA), Escherichia coli ATCC 25922, 15 and Pseudomonas aeruginosa ATCC 27853 could be spatiotemporally controlleD with light. Both molecular series 16 displayed a good activity winDow. The vancomycin Derivative DisplayeD excellent values against Gram-positive 17 strains after uncaging, and the next-generation caged cephalosporin derivative achieved good and broad activity 18 against both Gram-positive and Gram-negative strains after photorelease. 19 Key worDs: antibacterial agents, photopharmacology, photocaging, vancomycin, cephalosporin. 1 20 -

Physiological Probes & Assay Kits
Physiological Probes & Assay Kits Calcium Indicators · Membrane Potential Assays · pH Probes ® SIE HABEN DIE VISION, AAT Bioquest WIR HABEN DIE SUBSTANZ. Advancing Assay & Test Technologies www.aatbio.com Labeling Antibodies and Biopolymers Table of Contents Section 1 General Information 2 Section 6 Membrane Potential Measurement 33 Fast Response Membrane Potential Probes ................................................. 35 Section 2 Calcium Ion Detection 5 Slow Response Membrane Potential Probes ............................................... 36 Mitochondrial Membrane Potential Probes ................................................ 37 Fluo-8® Calcium Ion Indicators............................................................... ...... 8 FLIPR® Membrane Potential Assay Kits ....................................................... 38 2 Cal-520™ Calcium Ion Indicators .................................................................10 Rhod-4™ Calcium Ion Indicator ...................................................................11 Section 7 Index 39 Solutions Labeling Fluorescence Optimized BTC Calcium Ion Indicator ...........................................................................12 Fura-2 Calcium Ion Indicator .......................................................................12 Fura-8™ Calcium Ion Indicator ....................................................................12 Alphabetical Index .................................................................................. 40 Indo-1 Calcium Ion Indicator.......................................................................13 -

Specific Protein Labeling with Caged Fluorophores for Dual-Color Imaging and Super-Resolution Microscopy in Living Cells
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Specific protein labeling with caged fluorophores for dual-color imaging and super-resolution Cite this: Chem. Sci.,2017,8,559 microscopy in living cells† Sebastian Hauke,‡a Alexander von Appen,‡a Tooba Quidwai,b Jonas Riesb and Richard Wombacher*a We present new fluorophore-conjugates for dual-color photoactivation and super-resolution imaging inside live mammalian cells. These custom-designed, photo-caged Q-rhodamines and fluoresceins are cell-permeable, bright and localize specifically to intracellular targets. We utilized established orthogonal protein labeling strategies to precisely attach the photoactivatable fluorophores to proteins with subsequent activation of fluorescence by irradiation with UV light. That way, diffusive cytosolic proteins, histone proteins as well as filigree mitochondrial networks and focal adhesion proteins were visualized inside living cells. We applied the new photoactivatable probes in inverse fluorescence recovery after Creative Commons Attribution-NonCommercial 3.0 Unported Licence. photo-bleaching (iFRAP) experiments, gaining real-time access to protein dynamics from live biological Received 12th May 2016 settings with resolution in space and time. Finally, we used the caged Q-rhodamine for photo-activated Accepted 1st September 2016 localization microscopy (PALM) on both fixed and live mammalian cells, where the superior molecular DOI: 10.1039/c6sc02088g brightness and photo-stability directly resulted in improved localization precisions for different protein www.rsc.org/chemicalscience targets. Introduction photoactivation experiments inside living cells have been mainly restricted to the application of photo-modulatable FPs 11,12 This article is licensed under a For decades, photoactivation experiments, based on small so far.