View Full Page
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -

Floor Plate and Netrin-1 Are Involved in the Migration and Survival of Inferior Olivary Neurons
The Journal of Neuroscience, June 1, 1999, 19(11):4407–4420 Floor Plate and Netrin-1 Are Involved in the Migration and Survival of Inferior Olivary Neurons Evelyne Bloch-Gallego,1 Fre´de´ ric Ezan,1 Marc Tessier-Lavigne,2 and Constantino Sotelo1 1Institut National de la Sante´ et de la Recherche Me´ dicale U106, Hoˆ pital de la Salpeˆ trie` re, 75013 Paris, France, and 2Howard Hughes Medical Institute, Department of Anatomy, University of California at San Francisco, San Francisco, California 94143 During their circumferential migration, the nuclei of inferior oli- However, axons of the remaining olivary cell bodies located in vary neurons translocate within their axons until they reach the the vicinity of the floor plate still succeed in entering their target, floor plate where they stop, although their axons have already the cerebellum, but they establish an ipsilateral projection in- crossed the midline to project to the contralateral cerebellum. stead of the normal contralateral projection. In vitro experi- Signals released by the floor plate, including netrin-1, have ments involving ablations of the midline show a fusion of the been implicated in promoting axonal growth and chemoattrac- two olivary masses normally located on either side of the tion during axonal pathfinding in different midline crossing sys- ventral midline, suggesting that the floor plate may function as tems. In the present study, we report experiments that strongly a specific stop signal for inferior olivary neurons. These results suggest that the floor plate could also be involved in the establish a requirement for netrin-1 in the migration of inferior migration of inferior olivary neurons. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -
L4-Physiology of Motor Tracts.Pdf
: chapter 55 page 667 Objectives (1) Describe the upper and lower motor neurons. (2) Understand the pathway of Pyramidal tracts (Corticospinal & corticobulbar tracts). (3) Understand the lateral and ventral corticospinal tracts. (4) Explain functional role of corticospinal & corticobulbar tracts. (5) Describe the Extrapyramidal tracts as Rubrospinal, Vestibulospinal, Reticulospinal and Tectspinal Tracts. The name of the tract indicate its pathway, for example Corticobulbar : Terms: - cortico: cerebral cortex. Decustation: crossing. - Bulbar: brainstem. Ipsilateral : same side. *So it starts at cerebral cortex and Contralateral: opposite side. terminate at the brainstem. CNS influence the activity of skeletal muscle through two set of neurons : 1- Upper motor neurons (UMN) 2- lower motor neuron (LMN) They are neurons of motor cortex & their axons that pass to brain stem and They are Spinal motor neurons in the spinal spinal cord to activate: cord & cranial motor neurons in the brain • cranial motor neurons (in brainstem) stem which innervate muscles directly. • spinal motor neurons (in spinal cord) - These are the only neurons that innervate - Upper motor neurons (UMN) are the skeletal muscle fibers, they function as responsible for conveying impulses for the final common pathway, the final link voluntary motor activity through between the CNS and skeletal muscles. descending motor pathways that make up by the upper motor neurons. Lower motor neurons are classified based on the type of muscle fiber the innervate: There are two UMN Systems through which 1- alpha motor neurons (UMN) control (LMN): 2- gamma motor neurons 1- Pyramidal system (corticospinal tracts ). 2- Extrapyramidal system The activity of the lower motor neuron (LMN, spinal or cranial) is influenced by: 1. -

Neurochemical and Structural Organization of the Principal Nucleus of the Inferior Olive in the Human
THE ANATOMICAL RECORD 294:1198–1216 (2011) Neurochemical and Structural Organization of the Principal Nucleus of the Inferior Olive in the Human JOAN S. BAIZER,1* CHET C. SHERWOOD,2 PATRICK R. HOF,3 4 5 SANDRA F. WITELSON, AND FAHAD SULTAN 1Department of Physiology and Biophysics, University at Buffalo, Buffalo, New York 2Department of Anthropology, The George Washington University, Washington, District of Columbia 3Department of Neuroscience and Friedman Brain Institute, Mount Sinai School of Medicine, New York, New York 4Department of Psychiatry & Behavioural Neurosciences, Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada L8N 3Z5 5Department of Cognitive Neurology, University of Tu¨ bingen, Tu¨ bingen, Germany ABSTRACT The inferior olive (IO) is the sole source of the climbing fibers that innervate the Purkinje cells of the cerebellar cortex. The IO comprises several subdivisions, the dorsal accessory olive (DAO), medial accessory olive (MAO), and principal nuclei of the IO (IOpr); the relative sizes of these subnuclei vary among species. In human, there is an expansion of the cerebellar hemispheres and a corresponding expansion of the IOpr. We have examined the structural and neurochemical organization of the human IOpr, using sections stained with cresyl violet (CV) or immuno- stained for the calcium-binding proteins calbindin (CB), calretinin (CR), and parvalbumin (PV), the synthetic enzyme for nitric oxide (nNOS), and nonphosphorylated neurofilament protein (NPNFP). We found qualitative differences in the folding patterns of the IOpr among individuals and between the two sides of the brainstem. Quantification of IOpr volumes and indices of folding complexity, however, did not reveal consistent left–right differences in either parameter. -

Cerebellum and Inferior Olive
Cerebellum and Inferior Olivary Nucleus Spinocerebellum • Somatotopically organised (vermis controls axial musculature; intermediate hemisphere controls limb musculature) • Control of body musculature • Inputs… Vermis receives somatosensory information (mainly from the trunk) via the spinocerebellar tracts and from the spinal nucleus of V. It receives a direct projection from the primary sensory neurons of the vestibular labyrinth, and also visual and auditory input from brain stem nuclei. • Intermediate hemisphere receives somatosensory information (mainly from the limbs) via the spinocerebellar tracts (the dorsal spinocerebellar tract, from Clarke’s nucleus of the lower limb, and the cuneocerebellar tract, from the accessory cu- neate nucleus of the upper limb, carry information from muscle spindle afferents; both enter via the ipsilateral inferior cerebellar peduncle). • An internal feedback signal arrives via the ventral spinocerebellar tract (lower limb) and rostral spinocerebellar tract (upper limb). (Ventral s.t. decussates in the spinal cord and enters via the superior cerebellar peduncle, but some fibres re-cross in the cerebellum; rostral s.t. is an ipsilateral pathway and enters via sup. & inf. cerebellar peduncles.) • Outputs to fastigial nucleus, which projects to the medial descending systems: (1) reticulospinal tract [? n. reticularis teg- menti pontis and prepositus hypoglossi?]; (2) vestibulospinal tract [lateral and descending vestibular nn.]; and (3) an as- cending projection to VL thalamus [Å cells of origin of the ventral corticospinal tract]; (4) reticular grey of the midbrain [=periaqueductal?]; (5) inferior olive [medial accessory, MAO]. • … and interposed nuclei, which project to the lateral descending systems: (1) magnocellular portion of red nucleus [Å ru- brospinal tract]; (2) VL thalamus [Å motor cx which gives rise to lateral corticospinal tract]; (3) reticular nucleus of the pontine tegmentum; (4) inferior olive [dorsal accessory, DAO]; (5) spinal cord intermediate grey. -
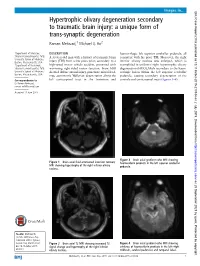
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

Holmes Tremor in Association with Bilateral Hypertrophic Olivary Degeneration and Palatal Tremor
Arq Neuropsiquiatr 2003;61(2-B):473-477 HOLMES TREMOR IN ASSOCIATION WITH BILATERAL HYPERTROPHIC OLIVARY DEGENERATION AND PALATAL TREMOR CHRONOLOGICAL CONSIDERATIONS Case report Carlos R.M. Rieder1, Ricardo Gurgel Rebouças2, Marcelo Paglioli Ferreira3 ABSTRACT - Hypertrophic olivary degeneration (HOD) is a rare type of neuronal degeneration involving the dento-rubro-olivary pathway and presents clinically as palatal tremor. We present a 48 year old male patient who developed Holmes’ tremor and bilateral HOD five months after brainstem hemorrhage. The severe rest tremor was refractory to pharmacotherapy and botulinum toxin injections, but was markedly reduced after thalamotomy. Magnetic resonance imaging permitted visualization of HOD, which appeared as a characteristic high signal intensity in the inferior olivary nuclei on T2- and proton-density-weighted images. Enlargement of the inferior olivary nuclei was also noted. Palatal tremor was absent in that moment and appears about two months later. The delayed-onset between insult and tremor following structural lesions of the brain suggest that compensatory or secondary changes in nervous system function must contribute to tremor genesis. The literature and imaging findings of this uncommon condition are reviewed. KEY WORDS: rubral tremor, midbrain tremor, Holmes’ tremor, myorhythmia, palatal myoclonus. Tremor de Holmes em associação com degeneração olivar hipertrófica bilateral e tremor palatal: considerações cronológicas. Relato de caso RESUMO - Degeneração olivar hipertrófica (DOH) é um tipo raro de degeneração neuronal envolvendo o trato dento-rubro-olivar e se apresenta clinicamente como tremor palatal. Relatamos o caso de um homem de 48 anos que desenvolveu tremor de Holmes e DOH bilateral cinco meses após hemorragia em tronco encefálico. -

Cerebellar Cortical Neuron Responses Evoked from the Spinal Border Cell Tract
Cerebellar cortical neuron responses evoked from the spinal border cell tract. Geborek, Pontus; Spanne, Anton; Bengtsson, Fredrik; Jörntell, Henrik Published in: Frontiers in Neural Circuits DOI: 10.3389/fncir.2013.00157 2013 Link to publication Citation for published version (APA): Geborek, P., Spanne, A., Bengtsson, F., & Jörntell, H. (2013). Cerebellar cortical neuron responses evoked from the spinal border cell tract. Frontiers in Neural Circuits, 7, [157]. https://doi.org/10.3389/fncir.2013.00157 Total number of authors: 4 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 04. Oct. 2021 ORIGINAL RESEARCH ARTICLE published: 08 October 2013 NEURAL CIRCUITS doi: 10.3389/fncir.2013.00157 Cerebellar cortical neuron responses evoked from the spinal border cell tract Pontus Geborek, Anton Spanne, Fredrik Bengtsson and Henrik Jörntell* Neural Basis of Sensorimotor Control, Department of Experimental Medical Science, Lund University, Lund, Sweden Edited by: Spinocerebellar systems are likely to be crucial for cerebellar hallmark functions such Egidio D’Angelo, University of Pavia, as coordination. -

External and Internal Modulation of the Olivo-Cerebellar Loop
REVIEW ARTICLE published: 19 April 2013 NEURAL CIRCUITS doi: 10.3389/fncir.2013.00073 In and out of the loop: external and internal modulation of the olivo-cerebellar loop Avraham M. Libster 1,2* and Yo s e f Ya ro m 1,2 1 Department of Neurobiology, Life Science Institute, Hebrew University, Jerusalem, Israel 2 Edmund and Lily Safra Center for Brain Sciences, Hebrew University, Jerusalem, Israel Edited by: Cerebellar anatomy is known for its crystal like structure, where neurons and connections Egidio D’Angelo, University of are precisely and repeatedly organized with minor variations across the Cerebellar Cortex. Pavia, Italy The olivo-cerebellar loop, denoting the connections between the Cerebellar cortex, Inferior Reviewed by: Olive and Cerebellar Nuclei (CN), is also modularly organized to form what is known as Gilad Silberberg, Karolinska Institute, Sweden the cerebellar module. In contrast to the relatively organized and static anatomy, the Deborah Baro, Georgia cerebellum is innervated by a wide variety of neuromodulator carrying axons that are State University, USA heterogeneously distributed along the olivo-cerebellar loop, providing heterogeneity to the *Correspondence: static structure. In this manuscript we review modulatory processes in the olivo-cerebellar Avraham M. Libster, Department loop. We start by discussing the relationship between neuromodulators and the animal of Neurobiology, Life Science Institute, Hebrew University, behavioral states. This is followed with an overview of the cerebellar neuromodulatory Silberman Building, Jerusalem signals and a short discussion of why and when the cerebellar activity should be 91904, Israel. modulated. We then devote a section for three types of neurons where we briefly review e-mail: [email protected] its properties and propose possible neuromodulation scenarios. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade.