Neurochemical and Structural Organization of the Principal Nucleus of the Inferior Olive in the Human
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Floor Plate and Netrin-1 Are Involved in the Migration and Survival of Inferior Olivary Neurons
The Journal of Neuroscience, June 1, 1999, 19(11):4407–4420 Floor Plate and Netrin-1 Are Involved in the Migration and Survival of Inferior Olivary Neurons Evelyne Bloch-Gallego,1 Fre´de´ ric Ezan,1 Marc Tessier-Lavigne,2 and Constantino Sotelo1 1Institut National de la Sante´ et de la Recherche Me´ dicale U106, Hoˆ pital de la Salpeˆ trie` re, 75013 Paris, France, and 2Howard Hughes Medical Institute, Department of Anatomy, University of California at San Francisco, San Francisco, California 94143 During their circumferential migration, the nuclei of inferior oli- However, axons of the remaining olivary cell bodies located in vary neurons translocate within their axons until they reach the the vicinity of the floor plate still succeed in entering their target, floor plate where they stop, although their axons have already the cerebellum, but they establish an ipsilateral projection in- crossed the midline to project to the contralateral cerebellum. stead of the normal contralateral projection. In vitro experi- Signals released by the floor plate, including netrin-1, have ments involving ablations of the midline show a fusion of the been implicated in promoting axonal growth and chemoattrac- two olivary masses normally located on either side of the tion during axonal pathfinding in different midline crossing sys- ventral midline, suggesting that the floor plate may function as tems. In the present study, we report experiments that strongly a specific stop signal for inferior olivary neurons. These results suggest that the floor plate could also be involved in the establish a requirement for netrin-1 in the migration of inferior migration of inferior olivary neurons. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -
L4-Physiology of Motor Tracts.Pdf
: chapter 55 page 667 Objectives (1) Describe the upper and lower motor neurons. (2) Understand the pathway of Pyramidal tracts (Corticospinal & corticobulbar tracts). (3) Understand the lateral and ventral corticospinal tracts. (4) Explain functional role of corticospinal & corticobulbar tracts. (5) Describe the Extrapyramidal tracts as Rubrospinal, Vestibulospinal, Reticulospinal and Tectspinal Tracts. The name of the tract indicate its pathway, for example Corticobulbar : Terms: - cortico: cerebral cortex. Decustation: crossing. - Bulbar: brainstem. Ipsilateral : same side. *So it starts at cerebral cortex and Contralateral: opposite side. terminate at the brainstem. CNS influence the activity of skeletal muscle through two set of neurons : 1- Upper motor neurons (UMN) 2- lower motor neuron (LMN) They are neurons of motor cortex & their axons that pass to brain stem and They are Spinal motor neurons in the spinal spinal cord to activate: cord & cranial motor neurons in the brain • cranial motor neurons (in brainstem) stem which innervate muscles directly. • spinal motor neurons (in spinal cord) - These are the only neurons that innervate - Upper motor neurons (UMN) are the skeletal muscle fibers, they function as responsible for conveying impulses for the final common pathway, the final link voluntary motor activity through between the CNS and skeletal muscles. descending motor pathways that make up by the upper motor neurons. Lower motor neurons are classified based on the type of muscle fiber the innervate: There are two UMN Systems through which 1- alpha motor neurons (UMN) control (LMN): 2- gamma motor neurons 1- Pyramidal system (corticospinal tracts ). 2- Extrapyramidal system The activity of the lower motor neuron (LMN, spinal or cranial) is influenced by: 1. -

Cerebellum and Inferior Olive
Cerebellum and Inferior Olivary Nucleus Spinocerebellum • Somatotopically organised (vermis controls axial musculature; intermediate hemisphere controls limb musculature) • Control of body musculature • Inputs… Vermis receives somatosensory information (mainly from the trunk) via the spinocerebellar tracts and from the spinal nucleus of V. It receives a direct projection from the primary sensory neurons of the vestibular labyrinth, and also visual and auditory input from brain stem nuclei. • Intermediate hemisphere receives somatosensory information (mainly from the limbs) via the spinocerebellar tracts (the dorsal spinocerebellar tract, from Clarke’s nucleus of the lower limb, and the cuneocerebellar tract, from the accessory cu- neate nucleus of the upper limb, carry information from muscle spindle afferents; both enter via the ipsilateral inferior cerebellar peduncle). • An internal feedback signal arrives via the ventral spinocerebellar tract (lower limb) and rostral spinocerebellar tract (upper limb). (Ventral s.t. decussates in the spinal cord and enters via the superior cerebellar peduncle, but some fibres re-cross in the cerebellum; rostral s.t. is an ipsilateral pathway and enters via sup. & inf. cerebellar peduncles.) • Outputs to fastigial nucleus, which projects to the medial descending systems: (1) reticulospinal tract [? n. reticularis teg- menti pontis and prepositus hypoglossi?]; (2) vestibulospinal tract [lateral and descending vestibular nn.]; and (3) an as- cending projection to VL thalamus [Å cells of origin of the ventral corticospinal tract]; (4) reticular grey of the midbrain [=periaqueductal?]; (5) inferior olive [medial accessory, MAO]. • … and interposed nuclei, which project to the lateral descending systems: (1) magnocellular portion of red nucleus [Å ru- brospinal tract]; (2) VL thalamus [Å motor cx which gives rise to lateral corticospinal tract]; (3) reticular nucleus of the pontine tegmentum; (4) inferior olive [dorsal accessory, DAO]; (5) spinal cord intermediate grey. -
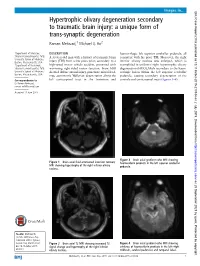
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

Holmes Tremor in Association with Bilateral Hypertrophic Olivary Degeneration and Palatal Tremor
Arq Neuropsiquiatr 2003;61(2-B):473-477 HOLMES TREMOR IN ASSOCIATION WITH BILATERAL HYPERTROPHIC OLIVARY DEGENERATION AND PALATAL TREMOR CHRONOLOGICAL CONSIDERATIONS Case report Carlos R.M. Rieder1, Ricardo Gurgel Rebouças2, Marcelo Paglioli Ferreira3 ABSTRACT - Hypertrophic olivary degeneration (HOD) is a rare type of neuronal degeneration involving the dento-rubro-olivary pathway and presents clinically as palatal tremor. We present a 48 year old male patient who developed Holmes’ tremor and bilateral HOD five months after brainstem hemorrhage. The severe rest tremor was refractory to pharmacotherapy and botulinum toxin injections, but was markedly reduced after thalamotomy. Magnetic resonance imaging permitted visualization of HOD, which appeared as a characteristic high signal intensity in the inferior olivary nuclei on T2- and proton-density-weighted images. Enlargement of the inferior olivary nuclei was also noted. Palatal tremor was absent in that moment and appears about two months later. The delayed-onset between insult and tremor following structural lesions of the brain suggest that compensatory or secondary changes in nervous system function must contribute to tremor genesis. The literature and imaging findings of this uncommon condition are reviewed. KEY WORDS: rubral tremor, midbrain tremor, Holmes’ tremor, myorhythmia, palatal myoclonus. Tremor de Holmes em associação com degeneração olivar hipertrófica bilateral e tremor palatal: considerações cronológicas. Relato de caso RESUMO - Degeneração olivar hipertrófica (DOH) é um tipo raro de degeneração neuronal envolvendo o trato dento-rubro-olivar e se apresenta clinicamente como tremor palatal. Relatamos o caso de um homem de 48 anos que desenvolveu tremor de Holmes e DOH bilateral cinco meses após hemorragia em tronco encefálico. -

External and Internal Modulation of the Olivo-Cerebellar Loop
REVIEW ARTICLE published: 19 April 2013 NEURAL CIRCUITS doi: 10.3389/fncir.2013.00073 In and out of the loop: external and internal modulation of the olivo-cerebellar loop Avraham M. Libster 1,2* and Yo s e f Ya ro m 1,2 1 Department of Neurobiology, Life Science Institute, Hebrew University, Jerusalem, Israel 2 Edmund and Lily Safra Center for Brain Sciences, Hebrew University, Jerusalem, Israel Edited by: Cerebellar anatomy is known for its crystal like structure, where neurons and connections Egidio D’Angelo, University of are precisely and repeatedly organized with minor variations across the Cerebellar Cortex. Pavia, Italy The olivo-cerebellar loop, denoting the connections between the Cerebellar cortex, Inferior Reviewed by: Olive and Cerebellar Nuclei (CN), is also modularly organized to form what is known as Gilad Silberberg, Karolinska Institute, Sweden the cerebellar module. In contrast to the relatively organized and static anatomy, the Deborah Baro, Georgia cerebellum is innervated by a wide variety of neuromodulator carrying axons that are State University, USA heterogeneously distributed along the olivo-cerebellar loop, providing heterogeneity to the *Correspondence: static structure. In this manuscript we review modulatory processes in the olivo-cerebellar Avraham M. Libster, Department loop. We start by discussing the relationship between neuromodulators and the animal of Neurobiology, Life Science Institute, Hebrew University, behavioral states. This is followed with an overview of the cerebellar neuromodulatory Silberman Building, Jerusalem signals and a short discussion of why and when the cerebellar activity should be 91904, Israel. modulated. We then devote a section for three types of neurons where we briefly review e-mail: [email protected] its properties and propose possible neuromodulation scenarios. -

Nervous System NS
NS Nervous System Course Goals Goals 1 . Understand the functional anatomy and physiology of the nervous system and their relation to therapeutic interventions. 2 . Understand the components of the neurologic exam, and demonstrate proficiency in performing a neurologic exam. 3 . Be able to identify the constellation of deficits that result from a lesion in the nervous system at the following loci: muscle, neuromuscular junction, spinal cord, brainstem, basal ganglia, cerebellum, cortex. 4 . Understand the major pathologic conditions that afflict the nervous system and recognize the basic principles of diagnosis and treatment for these disorders. 5 . Recognize the relationship between the structure of the nervous system and behavior. 6 . Understand the difference between behavioral neurology and psychiatry and recognize the different methods employed by each in the evaluation of behavioral disorders. 7 . Understand the dynamics of synaptic plasticity, and how changing structure leads to changes in behavior. 8 . Understand the overlap and differences between physical, pharmacologic, and behavioral manipulation of the nervous system. Nervous System Page 1 of 1 Rev 7/22/2019 Spring 2019 Nervous System Session Learning Objectives ADHD 1 . Describe approaches for identifying and describing attentional dysfunction, especially attentional dysfunction associated with attention deficit‐hyperactivity disorder. 2 . Describe the functional impact of attention deficit‐hyperactivity disorder. 3 . Describe at least 5 models for understanding why co‐morbidity with other psychiatric disorders is high for individuals with attention deficit‐hyperactivity disorder. 4 . State the likely need for long‐term treatment for individuals with attention deficit‐hyperactivity disorder. Alcoholics Anonymous 1 . Distinguish between spirituality and religion in 12 step programs 2 . -

A Patient with Bilateral Tremors Secondary to a Unilateral Brainstem Lesion: the Utility of Mollaret’S Triangle
Case Report Annals of Clinical Case Reports Published: 05 Jul, 2016 A Patient with Bilateral Tremors Secondary to a Unilateral Brainstem Lesion: The Utility of Mollaret’s Triangle Tran AT1*, Deep A2, Moguel-Cobos G2 and Lieberman A2 1Department of Neurology, U.S. Department of Veterans Affairs, USA 2Department of Neurology, Barrow Neurological Institute, USA Abstract A unilateral tremor developed in a patient’s left arm after a right midbrain hemorrhage. Thirteen years later, a re-bleed into that same area caused an additional right arm tremor. He now had bilateral arm tremors from a unilateral midbrain hemorrhage. The tremor was refractory to medications (propranolol, primidone, clonazepam, and levodopa). MRI brain showed bilateral hypertrophic olivary degeneration (HOD) from this unilateral midbrain hemorrhage. Although HOD has been associated with unilateral midbrain “rubral” tremor, it has not been described for bilateral intentional tremor. This case report illustrates how overlapping Mollaret’s triangles can explain this patient’s bilateral clinical findings. Keywords: Intentional tremor; Mollaret’s triangle; Midbrain tremor; Rubral tremor; Midbrain hemorrhage; Hypertrophic olivary degeneration Introduction Guillain-Mollaret’s triangle (GMT) is a commonly described anatomic model in association with palatal myoclonus [1]. It is also useful in the localization of tremor. The triangle consists of the dentate nucleus of the cerebellum, the red nucleus in the midbrain and the inferior olivary nucleus in the medulla. The central tegmental tract connects the red nucleus with the ipsilateral inferior olivary nucleus, while the superior cerebellar peduncle connects the dentate nucleus with OPEN ACCESS the contralateral red nucleus. The contralateral dentate nucleus and inferior olivary nucleus are *Correspondence: connected via the inferior cerebellar peduncle. -

Long-Term Depression of Glutamate-Induced Y-Aminobutyric
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 7386-7390, August 1993 Neurobiology Long-term depression of glutamate-induced y-aminobutyric acid release in cerebellum by insulin-like growth factor I (olivo-cerebeilar pathway/neuromodulation/electrical stimulation/microdialysis/pontine nudeus) MANUEL A. CASTRO-ALAMANCOS AND IGNACIO TORRES-ALEMAN Laboratories of Psychobiology and Cellular and Molecular Neuroendocrinology, Cajal Institute, Consejo Superior de Investigaciones Cientificas, Madrid, Spain Communicated by William T. Greenough, April 19, 1993 (received for review November 18, 1992) ABSTRACT We tested the possibility that insulin-like We decided to investigate whether IGF-I might be involved growth factor I (IGF-I) acts as a neuromodulator in the adult in the modulation of neurotransmitter function in the adult cerebellar cortex since previous observations indicated that cerebellar cortex since this area encompasses the climbing IGF-I is located in the olivo-cerebellar system encompassing the fiber terminal fields that synapse onto the dendritic arbors of inferior olive and Purkinje cells. We found that conjoint Purkinje cells. While Purkinje cells use y-aminobutyric acid administration ofIGF-I and glutamate through a microdialysis (GABA) as their main neurotransmitter, they receive a glu- probe stereotaxically implanted into the cerebellar cortex and tamatergic input from projecting granule cells through the deep cerebellar nuclei greatly depressed the release of Y-ami- parallel fiber system. In addition, Purkinje cells receive nobutyric acid (GABA), which normally follows a glutamate another excitatory input from inferior olivary neurons pulse. This inhibition was dose-dependent and long-lasting. through climbing fiber afferents (18). Moreover, the effect was specific for glutamate since KCI- Our working hypothesis was that IGF-I released by inferior induced GABA release was not modified by IGF-I. -

Connectivity of the Inferior Olivary Complex in Normal and Neonatally Hemicerebellectomized Rats
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 1980 Connectivity of the Inferior Olivary Complex in Normal and Neonatally Hemicerebellectomized Rats Rand S. Swenson Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Anatomy Commons Recommended Citation Swenson, Rand S., "Connectivity of the Inferior Olivary Complex in Normal and Neonatally Hemicerebellectomized Rats" (1980). Dissertations. 1981. https://ecommons.luc.edu/luc_diss/1981 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1980 Rand S. Swenson CONNECTIVITY OF THE INFERIOR OLIVARY COMPLEX IN NORMAL AND NEONATALLY HEMICEREBELLECTOMIZED RATS BY Rand S. Swenson A Dissertation Submitted to the Faculty of the Graduate School Of Loyola University of Chicago in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy February 1981 DEDICATION To my family, especially my wife, Mardrey Fish, my children, Krister and Ingrid, and my parents for their encouragement and patient understanding, this dissertation is lovingly dedicated. ii ACKNOWLEDGEMENTS The author takes this opportunity to thank Dr. Anthony J. Castro without whose patience, advice and guidance this dissertation would not have been completed . the members of his dissertation committee, Dr. John Disterhoft, Dr. James Hazlett, Dr. Robert Wurster and Dr. E. J. Neafsey for their helpful suggestions and criticisms. Miss Rebecca Auten for typing this manuscript. -

Inferior Olivary Nucleus Degeneration Does Not Lessen Tremor in Essential Tremor Elan D
Louis et al. Cerebellum & Ataxias (2018) 5:1 DOI 10.1186/s40673-018-0080-3 CASEREPORT Open Access Inferior Olivary nucleus degeneration does not lessen tremor in essential tremor Elan D. Louis1,2,3*, Daniel Trujillo Diaz1, Sheng-Han Kuo4, Shi-Rui Gan4,5, Etty P. Cortes6,7, Jean Paul G. Vonsattel6,7 and Phyllis L. Faust6 Abstract Background: In traditional models of essential tremor, the inferior olivary nucleus was posited to play a central role as the pacemaker for the tremor. However, recent data call this disease model into question. Case presentation: Our patient had progressive, long-standing, familial essential tremor. Upper limb tremor began at age 10 and worsened over time. It continued to worsen during the nine-year period he was enrolledinourbraindonationprogram(age85– 94 years), during which time the tremor moved from the moderate to severe range on examination. On postmortem examination at age 94, there were degenerative changes in the cerebellar cortex, as have been described in the essential tremor literature. Additionally, there was marked degeneration of the inferior olivary nucleus, which was presumed to be of more recent onset. Such degeneration has not been previously described in essential tremor postmortems. Despite the presence of this degeneration, the patient’s tremor not only persisted but it continued to worsen during the final decade of his life. Conclusions: Although the pathophysiology of essential tremor is not completely understood, evidence such as this suggests that the inferior olivary nucleus does not play a critical role in the generation of tremor in these patients. Keywords: Essential tremor, Cerebellum, Inferior olivary nucleus, Neurodegenerative, Purkinje cell, Pathology Background further support the central role that the cerebellum plays Essential tremor (ET) is one of the most common in ET pathogenesis [13–17].