Oceanic Squid Do fly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical Report No. 447 1974 •
FISHERIES RESEARCH BOARD OF CANADA TECHNICAL REPORT NO. 447 1974 • ... FISHERIES RESEARCH BOARD OF CANADA Technical Reports FRB Technical Reports are research documents that are of sufficient importance to be preserved, but which ·for some reason are not appropriate for primary scientific publication. No restriction is placed on subject matter and the series should reflect the broad research interests of FRB. These Reports can be cited in publications, but care should be taken to indicate their manuscript status. Some of the material in these Reports will eventually appear in the primary scientific literature. Inquiries concerning any particular Report should be directed to the issuing FRB establishment which is indicated on the title page. FISHERIES AND MARINE SERVICE TECHNICAL REPORT NO. 44 7 THE SQUID OF BRITISH COLUMBIA AS A POTENTIAL FISHERY RESOURCE - A PRELIMINARY REPORT by S.A. Macfarlane and M. Yamamoto Fisheries and Marine Service Vancouver Laboratory Vancouver, B.C. TABLE OF CONTENTS Page No. I. INTRODUCTION 1 II. BIOLOGICAL ASPECTS 3 III. COMMERCIAL ASPECTS 8 A. Fishing Methods 8 B. International Squid Fisher,y 15 c. Status of Squid in British Co1uabia 19 IV. NUTRITIONAL ASPECTS 27 v. PROCESSING 28 VI. DISCUSSION 30 VII. ACKNOWLEDGMENTS 32 VIII. REFERENCES 33 1. I. INTRODUCTION Available catch statistics from 1965 through 1971 indicate • that world-wide landings of squid totalled roughly 700,000 metric tons annually. An additional 100,000 metric tons of cuttlefish and about 160,000 metric tons of octopus were also landed annually. Apart from the well-established squid fishery in the Monterey area of California and the relatively minor inshore squid fishery off Newfoundland, the North American fishing industry has tended to ignore the possibility of further exploitation and utilization of this resource. -

Vertical Distribution of Pelagic Cephalopods *
* Vertical Distribution of Pelagic Cephalopods CLYDE F. E. ROPER and RICHARD E. YOUNG SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. These pub- lications are distributed by mailing lists to libraries, laboratories, and other interested institutions and specialists throughout the world. Individual copies may be obtained from the Smithsonian Institution Press as long as stocks are available. S. DILLON RIPLEY Secretary Smithsonian Institution SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 Vertical Distribution of Pelagic Cephalopds Clyde F. -

Cephalopod Beak Sections Used to Trace
Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, Jim Roberts, Eduarda Pereira, José Xavier To cite this version: José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, et al.. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study. Marine Environmental Research, Elsevier, 2020, 161, pp.105049. 10.1016/j.marenvres.2020.105049. hal-02904190 HAL Id: hal-02904190 https://hal.archives-ouvertes.fr/hal-02904190 Submitted on 21 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José P. Queirós*a, Paco Bustamanteb,c, Yves Chereld, João P. Coelhoe, José Secof,g, Jim Robertsh, Eduarda Pereiraf & José C. Xaviera,i a – University of Coimbra, MARE – Marine and Environmental Sciences Centre, Department of Life Sciences, 3000-456, Coimbra, Portugal b - Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS-La Rochelle Université, 2 rue Olympe de Gouges, 17000 La Rochelle, France c - Institut Universitaire de France (IUF), 1 rue Descartes 75005 Paris, France d - Centre d’Etudes Biologiques de Chizé, UMR 7372 du CNRS–La Rochelle Université, 79360 Villiers–en–Bois, France. -

University of Groningen Sperm Storage and Mating in the Deep-Sea
University of Groningen Sperm storage and mating in the deep-sea squid Taningia danae Joubin, 1931 (Oegopsida Hoving, Hendrik Jan T.; Lipinski, Marek R.; Videler, John J.; Bolstad, Kat S. R. Published in: Marine Biology DOI: 10.1007/s00227-009-1326-7 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2010 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Hoving, H. J. T., Lipinski, M. R., Videler, J. J., & Bolstad, K. S. R. (2010). Sperm storage and mating in the deep-sea squid Taningia danae Joubin, 1931 (Oegopsida: Octopoteuthidae). Marine Biology, 157(2), 393- 400. https://doi.org/10.1007/s00227-009-1326-7 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Fao Species Identification Sheets for Fishery Purposes
3D FAO SPECIES IDENTIFICATION SHEETS FOR FISHERY PURPOSES WESTERN CENTRAL ATLANTIC (Fishing Area 31) edited by W. Fischer Marine Resources Service Fishery Resources and Environment Division FAO Fisheries Department This publication has been prepared and printed with the support of the UNDP/FAO International Project for the Development of Fisheries in the Western Central Atlantic VOLUME VI CONTENTS: Lobsters Shrimps and Prawns True Crabs Stomatopods Bivalves Gastropods Chitons * Cephalopoda + Sea Turtles FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1978 Bibliographic Reference: Roper, C.F.E. In: Fi.ch«, w. CEO cms) Rome, FAO, pag.var. FAO species identification sheets for fishery purposes. Western Central Atlantic (fishing area 31>. Vols. 1-7 Identification sheets. Taxonomy. Geographic distribution. Fisheries. Vernacular names. Bony fishes. Sharks. Batoid fishes. Lobsters. Shrimps. True crabs. S tomato pods. Molluscs. Sea turtles. ASH. FAO Sheets Fishing Area 3 I TECHNICAL TERMS lamellae I hectocotylus (or modified •< portion) suckers buccal membrane funnel groove oegopsid eye normal sucker: funne I funnel-mantle fusion funnel locking cartilage mantle locking cartilage 1 dactylus ~T"Y fin (posteriorly concave) _ fin (posteriorly V t convex) loss t— fin length example of hectocotylized arm in male (Illex ittecebrosue) __cail arm I (dorsal) mantle length buccal lappet J a composite diagram illustrating beak jaws basic squid (teuthoid) features ventral view buccal suckers buccal connective (ventrally attached) buccal connective (dorsally attached arm IV (ventral) diagram of oral surface of brachial crown and buccal membrane oval with inward simple, -^-shaped J_-shaped subtriangular projecting knobs straight basic types of funnel locking cartilage (cartilaginous grooves that lock with corresponding ridges on inner mantle wall to keep base of funnel in position during water expulsion) - 2 - FAO Sheets CEFHALOPOOS Fishing Area 31 mantle Length lame 1Lae (internal) / funnel aperture suckers V" total length A. -
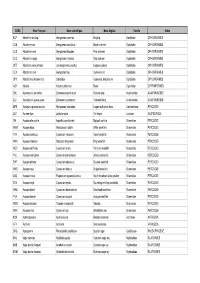
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

A Dietary Study of Moroteuthis Ingens and Other Southern Ocean Squid Species: Combined Stomach Contents and Fatty Acid Analyses
A dietary study of Moroteuthis ingens and other Southern Ocean squid species: combined stomach contents and fatty acid analyses by Katrina L. Phillips Bachelor of Science (Biochemistry, Zoology), University of Tasmania Bachelor of Antarctic Studies (Honours), University of Tasmania Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy University of Tasmania (October, 2003) ii "EMBRIAGADO por la evidencia del prodigio, en aquel momenta se olvid6 de la frustraci6n de sus empresas delirantes y del cuerpo de Melquiades abandonado al apetito de los calamares." "INTOXICATED by the evidence of the miracle, he forgot at that moment about the frustration of his delirious undertakings and Melquiades' body, abandoned to the appetite of the squids." Gabriel Garcia Marquez Cien aiios de soledad (One hundred years of solitude) (1967) "No shortage of explanations for life's mysteries. Explanations are two a penny these days. The truth, however, is altogether harder to find." Salman Rushdie The ground beneath her feet (1999) iii This thesis contains no material which has been accepted for a degree or diploma by the Unjversity or any other institution, except by way of background information and which has been duly acknowledged, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgment has been made in the text. Thjs thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968. Katrina L. Phillips ABSTRACT The squid fauna are a key component of the Southern Ocean ecosystem, although unfortunately little is known of their distribution, biology and ecology. -
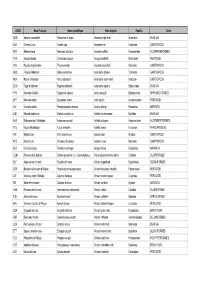
Liste Espèces
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

ปลาหมึกกระดองเข็ม ชื่อวิทยาศาสตร์ Sepia Kobiensis Hoyle, 1885 ชื่อท้องถิ่น ปลาหมึกกระดองเข็ม ชื่อสามัญ Kobi Cuttlefish แหล่งที่พบครั้งแรก ตลาดโยโกฮามา ประเทศญี่ปุ่น
ºÑÞªÕÃÒ¡Ò÷ÃѾÂҡêÕÇÀÒ¾ÁÍÅÅÑÊ¡Òã¹»ÃÐà·Èä·Â ห มึก บทนำ� ความหลากหลายทางชีวภาพได้แก่ความหลากหลายของสปีชีส์ พันธุกรรม และระบบนิเวศของสิ่งมีชีวิต ในแต่ละชีวมณฑล ที่ได้อุบัติขึ้นบนดาวพระเคราะห์โลกแห่งนี้มาหลายพันล้านปีแล้ว และได้กลายเป็นทรัพยากร ที่ส�าคัญยิ่ง ที่ผสมกลมกลืนกันจนกลายเป็น “โลกสีเขียว The Green Planet” มาจวบจนถึงปัจจุบันนี้ และชีวิต ที่ถือว่าอุบัติมาจนถึงสูงสุดในเวลานี้คือสปีชีส์ที่เรียกว่า “มนุษย์ Homo sapiens” มนุษย์ได้อยู่ผสมกลมกลืนกับ ชีวิตอื่นๆ ได้สร้างสรรค์ เบียดเบียน และท�าลายล้าง ตามวิถีทางของความหลากหลาย และก�าลังเปลี่ยนแปลงไปตาม แนวทางแห่งวิวัฒนาการตามกาลเวลาและสถานที่ ประเทศไทยตั้งอยู่บนท�าเลที่บรรพบุรุษเรียกกันว่า “สุวรรณภูมิ” อยู่บนพื้นที่ที่มีความพอดีหลายอย่าง ทั้งสภาพภูมิประเทศที่มีระบบนิเวศแทบทุกระบบ ภูมิอากาศที่ดี ตั้งอยู่บริเวณตอนกลางของคาบสมุทรอินโดจีน ระหว่างละติจูด 5 องศา 37 ลิปดาเหนือ กับ 20 องศา 27 ลิปดาเหนือ และระหว่างลองจิจูด 97 องศา 22 ลิปดา ตะวันออก กับ 105 องศา 37 ลิปดาตะวันออก หรือบริเวณซีกโลกเหนือในเขตละติจูดต�่า ระหว่างเส้นศูนย์สูตรกับ เส้นทรอปิกออฟเคนเซอร์นั่นเอง จึงจัดอยู่ในประเทศเขตร้อนเหมาะสมต่อวิถีเขตร้อนที่น�าไปสู่ความเจริญมั่งคั่งของ พืชพันธุ์ธัญญาหารและสมบูรณ์แบบของปัจจัยสี่ บรรพบุรุษของไทยจึงได้กล่าวเป็นปริศนาให้ลูกหลานได้รู้อย่าง ต่อเนื่องว่า “สยามประเทศแห่งนี้เต็มไปด้วยทรัพย์ในดินสินในน�้า” “ในน�้ามีปลาในนามีข้าว” คนไทยในอดีตได้ซาบซึ้ง ในสิ่งเหล่านี้ หากแต่ว่าโลกาภิวัตน์ในเวลาต่อมาได้ท�าให้ภูมิคุ้มกันของคนไทยอ่อนลงจากอิทธิพลความคิดของ ต่างชาติ ท�าให้ลืมรากเหง้าฐานแห่งสยามประเทศเกือบจะสิ้นเชิง ความตระหนักในทรัพย์ในดินสินในน�้าจึงเสื่อมถอยลง ดังนั้นจึงมีความจ�าเป็นต้องเรียกความตระหนักเหล่านี้กลับมาก่อนที่จะสายไปมากกว่านี้ -

Lischka Et Al 2020 MER.Pdf
Influence of sexual dimorphism on stable isotopes and trace element concentrations in the greater hooked squid Moroteuthopsis ingens from New Zealand waters A. Lischka, H. Braid, Y. Cherel, K. Bolstad, T. Lacoue-Labarthe, P. Bustamante To cite this version: A. Lischka, H. Braid, Y. Cherel, K. Bolstad, T. Lacoue-Labarthe, et al.. Influence of sexual dimor- phism on stable isotopes and trace element concentrations in the greater hooked squid Moroteuthopsis ingens from New Zealand waters. Marine Environmental Research, Elsevier, 2020, 159, pp.104976. 10.1016/j.marenvres.2020.104976. hal-02546145 HAL Id: hal-02546145 https://hal.archives-ouvertes.fr/hal-02546145 Submitted on 17 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Influence of sexual dimorphism on stable isotopes and trace element concentrations in the greater hooked squid Moroteuthopsis ingens from New Zealand waters A. Lischka1, H. Braid1, Y. Cherel2, K. Bolstad1, T. Lacoue-Labarthe3, P. Bustamante3, 4 1AUT Lab for Cephalopod Ecology & Systematics, School of Science, Auckland University -

Kunci Determinasi
TAKSONOMI HEWAN Gunaria Siagian, S.Pd., M.Si TAKSONOMI HEWAN Penulis: Gunaria Siagian Desain Cover: Ridwan Tata Letak: Atep Jejen Proofreader: Elin Marlina ISBN: 978-623-6608-59-3 Cetakan Pertama: November, 2020 Hak Cipta 2020, Pada Penulis Hak Cipta Dilindungi Oleh Undang-Undang Copyright © 2020 by Penerbit Widina Bhakti Persada Bandung All Right Reserved Dilarang keras menerjemahkan, memfotokopi, atau memperbanyak sebagian atau seluruh isi buku ini tanpa izin tertulis dari Penerbit. PENERBIT: WIDINA BHAKTI PERSADA BANDUNG Komplek Puri Melia Asri Blok C3 No. 17 Desa Bojong Emas Kec. Solokan Jeruk Kabupaten Bandung, Provinsi Jawa Barat Anggota IKAPI Cabang Jawa Barat No. 360/JBA/2020 Website: www.penerbitwidina.com Instagram: @penerbitwidina Email: [email protected] PRAKATA asa syukur yang teramat dalam dan tiada kata lain yang patut kami ucapkan selain mengucap rasa syukur. Karena berkat rahmat dan R karunia Tuhan Yang Maha Esa, buku yang berjudul “Biologi Umum” telah selesai di susun dan berhasil diterbitkan, semoga buku ini dapat memberikan sumbangsih keilmuan dan penambah wawasan bagi siapa saja yang memiliki minat terhadap pembahasan tentang Biologi Akan tetapi pada akhirnya kami mengakui bahwa tulisan ini terdapat beberapa kekurangan dan jauh dari kata sempurna, sebagaimana pepatah menyebutkan “tiada gading yang tidak retak” dan sejatinya kesempurnaan hanyalah milik tuhan semata. Maka dari itu, kami dengan senang hati secara terbuka untuk menerima berbagai kritik dan saran dari para pembaca sekalian, hal tersebut tentu sangat diperlukan sebagai bagian dari upaya kami untuk terus melakukan perbaikan dan penyempurnaan karya selanjutnya di masa yang akan datang. Terakhir, ucapan terima kasih kami sampaikan kepada seluruh pihak yang telah mendukung dan turut andil dalam seluruh rangkaian proses penyusunan dan penerbitan buku ini, sehingga buku ini bisa hadir di hadapan sidang pembaca.